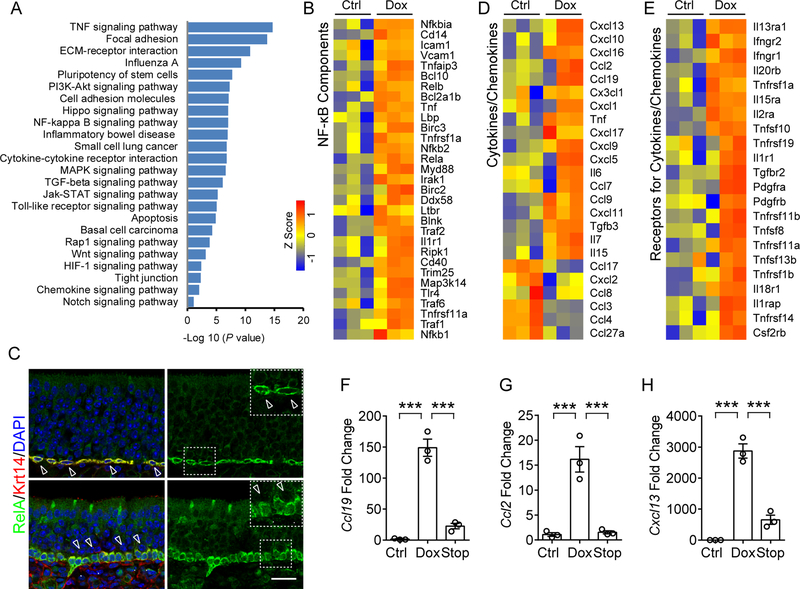
Figure 2. NF-κB activation in HBC targeted broad spectrum cytokines and chemokines.
(A) Pathway enrichment analysis of genes that were significantly upregulated (>2-fold, p < 0.05) in sorted HBCs after 6 w Dox treatment, relative to untreated control. Enriched pathways were presented according to –log10 (p value).
(B) Heat map of NF-κB pathway-related genes that are significantly upregulated in HBCs after Dox treatment.
(C) Immunostaining analysis of RelA localization in HBCs. RelA is highly expressed in Krt14+ HBCs and largely absent in nuclei (upper panel, hollow arrow heads) in the static state. Upon Dox-initiated inflammation, RelA translocated to the nucleus in Krt14+ HBCs (Arrow heads in lower panel, compared to hollow arrow heads). IOI mouse was treated with Dox for 12 days.
(D) Heat map of cytokines or chemokines genes that are differentially expressed in HBCs.
(E) Heat map of upregulated receptor genes of cytokines or chemokines in HBCs. The color scale shows the Z score of each gene.
(F–H) qPCR analysis of mRNA expression of Ccl19 (F), Ccl2 (G), and Cxcl13 (H) in HBCs sorted from control, Dox treated and 3 days post-Dox treatment (Stop) mice.
Data are represented as mean ± SEM, n = 3 independent samples. ***P < 0.001, P values were calculated by one-way ANOVA. Scale bar, 50 μm. See also Figure S2.