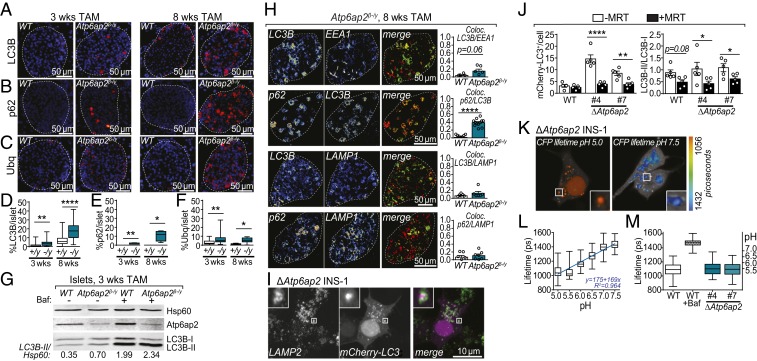
Fig. 5.
Atp6ap2 deletion induces the formation of large vacuoles without impairment of autophagy or lysosomal acidification. (A–C) Immunostaining of pancreatic sections from WT and Atp6ap2β-/y mice for LC3B (A), p62 (B), and ubiquitin (C) after 3 and 8 wk of TAM treatment. All proteins are depicted in red with a nuclear stain (DAPI) shown in blue. White dashed line indicates total islet area. (D–F) Quantification of A–C of the % area of positive staining within total islet area. WT are “+/y”; Atp6ap2β-/y as “−/y”. (G) Islets isolated from WT and Atp6ap2β-/y mice treated with TAM for 3 wk were cultured ±100 nM Bafilomycin A1 (Baf) for 120 min and then analyzed by Western blotting for expression of LC3B-I, LC3B-II, and Atp6ap2. The ratio of LC3B-II to loading control (Hsp60) is shown. Result is representative of 2 independent experiments. (H) Colocalization analysis of LC3B, EEA1, p62, and LAMP1 by immunofluorescence confocal microscopy of WT and Atp6ap2β-/y islets treated with TAM for 8 wk. Representative images of Atp6ap2β-/y islets are shown with quantitation of colocalization (Pearson’s). (I) Colocalization analysis of LC3B and LAMP2 by confocal microscopy of ΔAtp6ap2 INS-1 cells transfected with mCherry-LC3B. (J) WT and ΔAtp6ap2 INS-1 cells were transfected with mCherry-LC3B and incubated ±1 μM MRT 68921 for 12 h. mCherry-LC3B+ structures were counted (Left) and the ratio of LC3B-II/LC3B-I levels determined by Western blotting (Right). (K and L) LC3B+-vacuolar pH measurements in ΔAtp6ap2 INS-1 cells by FLIM with calibration to nigericin-buffered solutions from pH 5.0 to 7.5. (M) Vacuole pH measurements of WT and 2 independent ΔAtp6ap2 INS-1 clones were identical while addition of Baf increased vacuolar pH. For all panels, *P < 0.05; **P < 0.01; ****P < 0.0001.