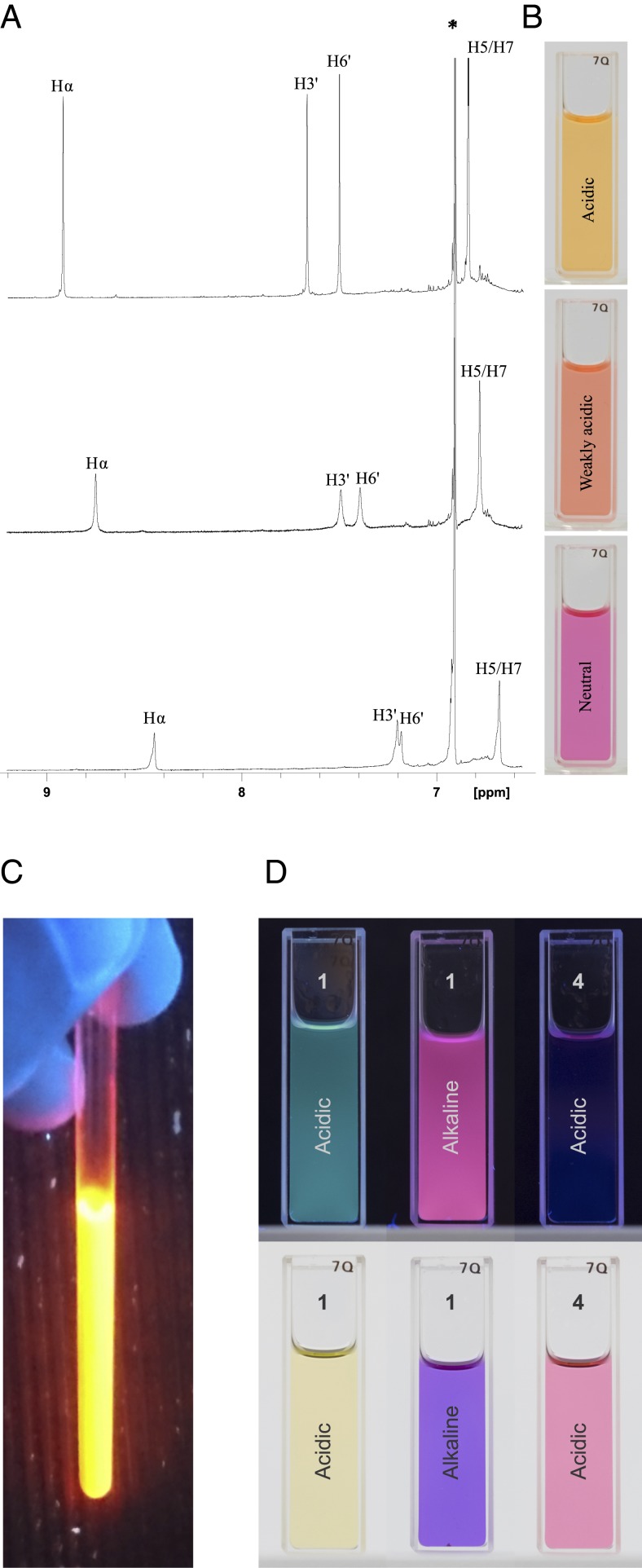
Fig. 3.
1H NMR spectra, colors, and fluorescence of auronidin 4-neohesperidoside from Marchantia in solvents with different acidities, demonstrating lack of some typical anthocyanin properties. (A and B) Auronidin 4-neohesperidoside (1) dissolved in methanol containing various amounts of TFA. The aromatic regions of the corresponding 1H NMR spectra (A) show that the color changes from orange to pink-purple (B) are in accordance with increased amounts of the anionic form of 1 in an acid-base equilibrium when the acid content is reduced. The lack of additional peaks in the NMR spectra prove the absence of various anthocyanin equilibrium forms expressing different colors. (C) Auronidin 4-neohesperidoside dissolved in deuterated dimethyl sulfoxide containing 5% TFA in 365-nm UV light. (D) Comparison of auronidin 4-neohesperidoside and the anthocyanin cyanidin 3-glucoside dissolved in methanol containing 0.5% TFA (acidic) or 0.5% 0.2 M NaOH (alkaline). The samples were illuminated by a 365-nm UV lamp (Top) or ambient light (Bottom). The anthocyanin in alkaline is not shown, as it degraded in that solvent.