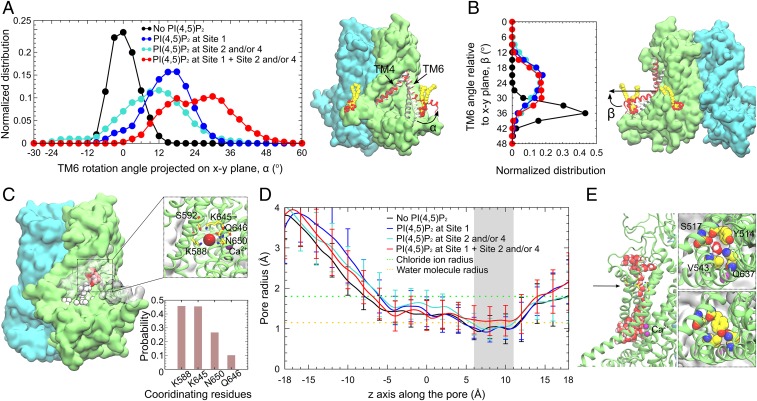
Fig. 8.
Conformational changes induced by PI(4,5)P2 binding. (A) Rotation angle of the cytoplasmic portion of TM6 with respect to its position in the cryo-EM structure, projected onto the x–y plane. (A, Left) Distribution of the rotation angle normalized over the 600-ns full-length lipid simulations for each palmitoyl-oleoyl PI(4,5)P2 occupancy state. The cytoplasmic portion of TM6 fluctuates around its initial position (0°) in the absence of PI(4,5)P2 (black). Single occupancy of site 1 (blue), or single/double occupancy of sites 2 and 4 (cyan) shifts the rotation angle to positive values (cytoplasmic portion of TM6 away from the pore). Multiple occupancy of PI(4,5)P2 at sites 1 plus 2 and/or 4 (red) rotates TM6 most dramatically away from the pore. (A, Right) Snapshots of TM6 in the multiply occupied state (red), the PI(4,5)P2-free state (black), and the cryo-EM structure (white). Bound PI(4,5)P2 is shown in van der Waals (vdW) representation. (B, Left) Distribution of the angle between the TM6 cytoplasmic portion and the x–y plane. (B, Right) Snapshot showing the angle relative to the membrane in the multiple-occupied state (red), the PI(4,5)P2-free state (black), and the cryo-EM structure (white). (C) Time series snapshots showing the spontaneous penetration of Cl− ions (vdW spheres, white-to-red over time) in the multiply occupied state. Coordination of the Cl− ion deeply binding inside the pore is shown in the Inset, with the probability of coordination for all of the binding events shown below. Cl− and Ca2+ ions are shown as red and purple spheres, respectively. (D) Average radius of the ion conduction pore calculated using HOLE (86) illustrates a bottleneck between 6 and 11 Å (gray shading). The bottleneck in the multiply occupied state dilates ∼30% compared to other states. Orange dotted line: radius of water molecule (1.15 Å). Green dotted line: radius of Cl− ion (1.8 Å). (E, Left) Hydration of the ion conduction pore when the bottleneck (black arrow) dilates in the multiply occupied state. (E, Right) Top views of the pore showing the amino acids forming the bottleneck. The side-chain orientation of Y514 affects the pore radius and hydration.