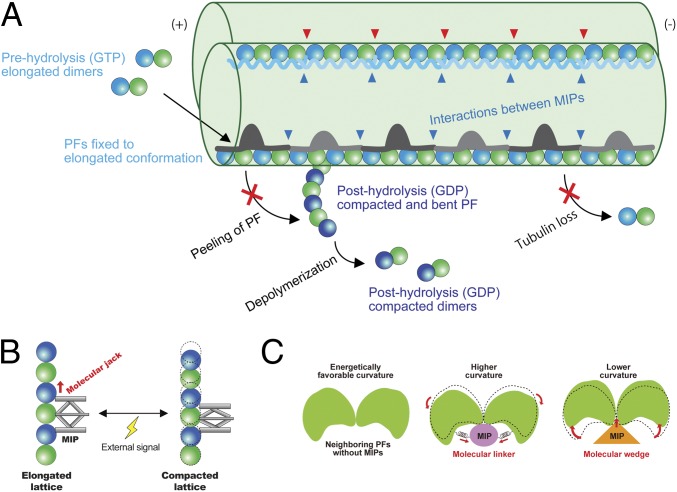
Fig. 6.
Model of stabilization mechanisms of the doublet tubulin lattice by MIPs. (A) Model of the impacts of the MIPs on the doublet. First, elongated tubulin dimers in GTP prehydrolysis state are incorporated into the tubulin lattice. This elongated and stable conformation is fixed after assembly into the lattice through the interactions with the MIPs. The network of MIPs (blue arrowheads) also holds the tubulin lattice from the inside to prevent the loss of tubulin or breakage. At the plus end, MIPs prevent the peeling of PFs and depolymerization by keeping PFs in a stable and elongated conformation. Hence, the doublet is stabilized by the MIPs at several different levels to ensure that it can withstand the mechanical stress and prevent catastrophic events for the cilia. Some MIPs, such as Rib43a, have insertions into the tubulin lattice (red arrowheads), causing the larger interdimer gap and bimodal dimer distance. (B) Schematic diagram of the function of the MIPs in regulating tubulin lattice length. Some MIPs work as a molecular jack to keep the tubulin lattice elongated. External signals could change the MIP property and thereby the tubulin lattice. (C) MIPs regulate the angles between PFs. Without MIPs, tubulin lattice takes an energetically favorable curvature. Some MIPs work as molecular linkers, which hold adjacent tubulin pairs together so that it will take a higher curvature such as in the PFs A9/A10. Other MIPs, in particular, Rib43a work as molecular wedges and open the PF pairs and induce a lower curvature.