Fig. 5.
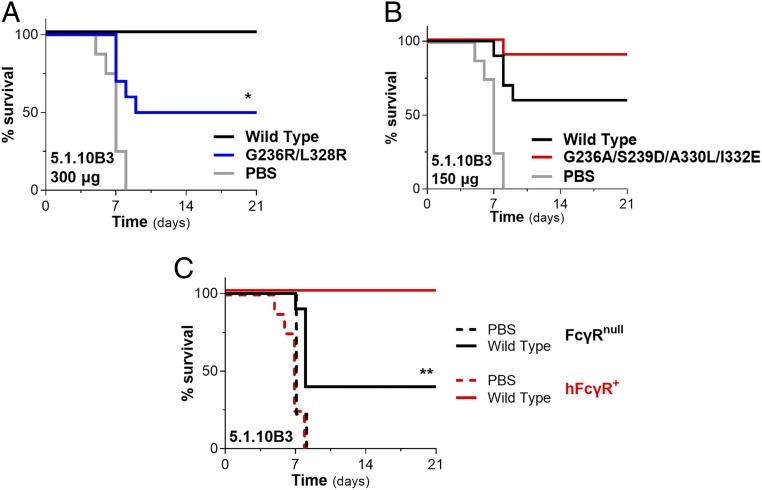
Antibodies against the GP chalice bowl require FcγR engagement for in vivo antiviral activity. The capacity of the anti-GP mAb 5.1.10B3, which targets the chalice bowl region of GP1 (GP1 head/glycan cap interface) to protect against EBOV infection, was evaluated in FcγR humanized (hFcγR+) mice. Titration studies established the optimal and suboptimal mAb dose required for protection (SI Appendix, Fig. S4F). (A) Comparison of the in vivo protective activity of 5.1.10B3 wild-type human IgG1 and Fc variant (GRLR) with diminished FcγR binding affinity. mAb (300 μg) was administered intraperitoneally 1 d prior to challenge with mouse-adapted EBOV. (B) Using a suboptimal mAb dose (150 μg), the antiviral potency of 5.1.10B3 expressed as either wild-type human IgG1 or Fc engineered (GASDALIE variant) for enhanced FcγR affinity was evaluated in vivo. (C) Follow-up experiments to confirm the contribution of Fc–FcγR interactions to the in vivo protective activity of the 5.1.10B3 mAb were performed using mouse strains deficient for FcγRs. The antiviral activity of 5.1.10B3 (wild-type human IgG1; 300 μg) was evaluated in FcγR-deficient (FcγRnull) and hFcγR+ mice; n = 9 to 10 per experimental group. Log rank (Mantel–Cox) test. *P = 0.0113 wild type vs. GRLR; **P = 0.0046 wild-type 5.1.10B3-treated FcγRnull vs. hFcγR+ mice.