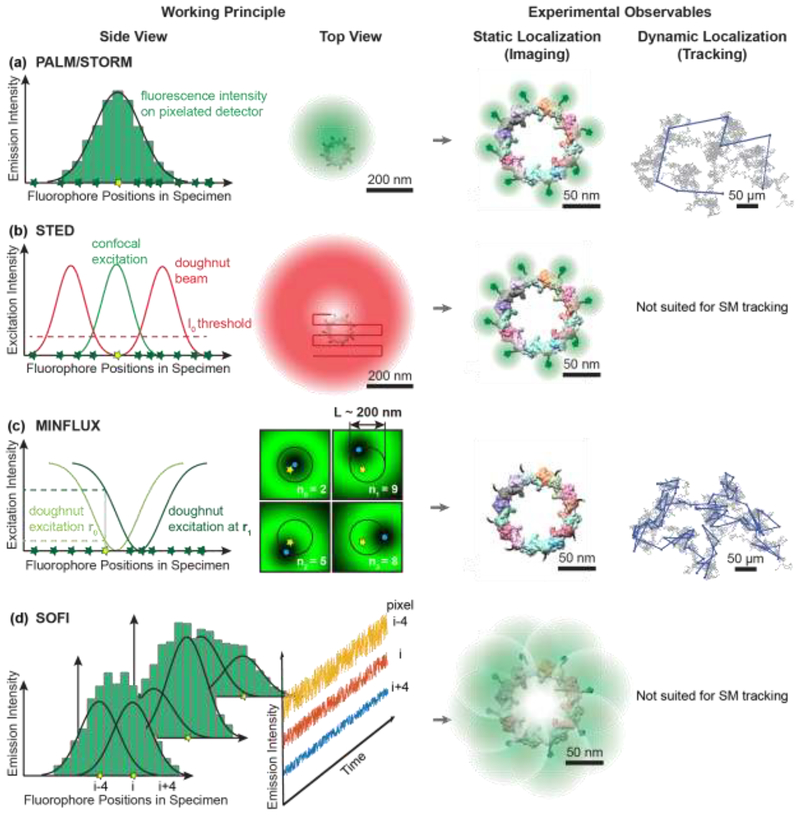
Figure 1. Working principles of selected super-resolution imaging modalities and their resolving capabilities.
a) In coordinate-stochastic (F)PALM/STORM microscopy, fluorophores randomly switch between fluorescence ON and OFF states (yellow and green stars, respectively) when illuminated by wide-field excitation light, resulting in isolated fluorescence signals on the detector. The positions of individual molecules are determined by fitting the pixelated (diffraction-limited) single-molecule images (green histogram) with a mathematical model (black line). Localization precisions of 20–80 nm are routinely achieved for camera-based single-molecule imaging in living cells as indicated by the green probability densities. If a molecule emits photons during several camera frames, its position can be determined at subsequent time points in single-molecule tracking applications, as indicated by the blue displacements overlaid on the simulated Brownian diffusion trajectory (grey).b) In coordinate-targeted STED microscopy, fluorophores (green and yellow stars) in the specimen are selectively excited by a conventional confocal excitation beam (green) and then forced back to the ground state by a red-shifted doughnut beam (red) through stimulated emission. STED microscopy provides a smaller probing volume compared to confocal microscopy, because only the fluorophores near the center of the doughnut beam (below the I0 intensity threshold for stimulated emission, dashed line) are allowed to remain in the excited state and fluoresce (yellow star). A super-resolved image is acquired pixel-by-pixel by scanning the excitation beam and the overlapping STED doughnut through the sample. STED can achieve a resolution of 20–50 nm in whole-cell imaging, as indicated by the green fluorescence signals overlaid on fluorescently-labeled nucleoporin proteins of the nuclear pore complex [61,62]. The deterministic scanning pattern of STED is not suited for single-molecule tracking; nevertheless, tracking of synaptic vesicles labeled with multiple fluorophores has been demonstrated [63]. c) MINFLUX microscopy is a hybrid of coordinate-targeted and coordinate-stochastic microscopy. Wide-field illumination is used to randomly photoactivate a single fluorophore in the specimen, and then a doughnut beam is targeted to four predefined coordinates (blue dots) around the fluorophore. If the fluorophore is located near the intensity minimum, it will only emit a few fluorescence photons, and any small displacement of the doughnut will cause it to emit more photons. The position of the fluorescence emitter can be calculated from the number of photons emitted at the four targeted coordinates (n0, n1, n2, n3). Localization precisions of 2 nm can be achieved for MINFLUX single-molecule imaging, as indicated by the green probability densities, and 20–50 nm for MINFLUX single-molecule tracking in living cells, as indicated by the blue displacements. d) SOFI relies on stochastic fluctuations of fluorescence emitters that independently transition between fluorescent and non-fluorescent states over time. If these emitters are positioned closer than the diffraction limit, then the signal in a given pixel (i-4, i, or i+4) is the sum of the fluorescence signals (overlapping Gaussian profiles) of multiple emitters (yellow stars). Due to fluorophore blinking, the intensity measured in each pixel fluctuates in time (blue, red, and yellow profiles). Computing the nth-order auto- and cross-cumulants of the temporal fluctuations in each pixel can provide resolution improvements by up to a factor of n. Resolutions on the order of 100 nm are routinely achieved in living cells, as indicated by the green probability densities.