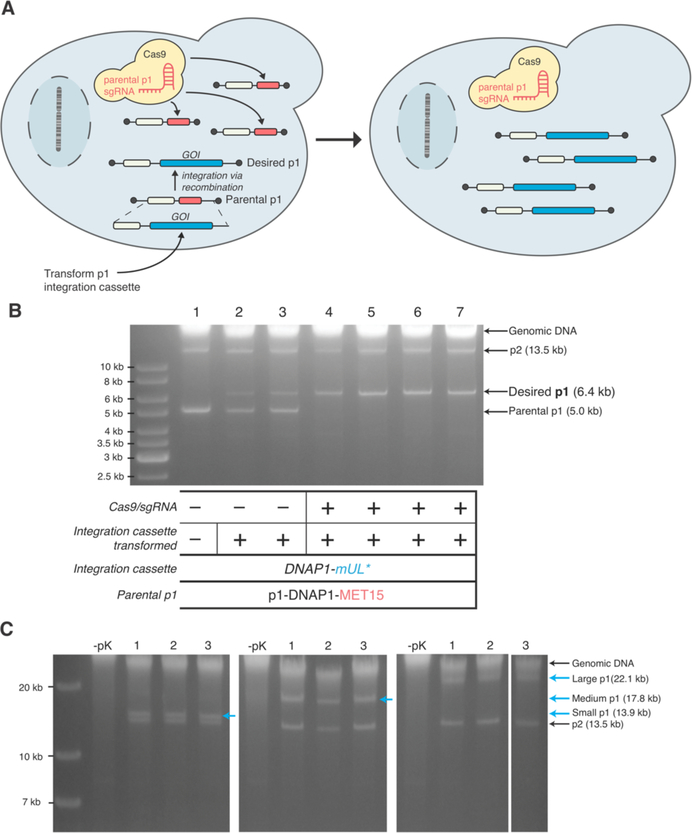
Figure 3—
(A) Accelerating genetic manipulation of p1 with CRISPR/Cas9. After transformation of an integration cassette containing a GOI and a selectable marker, transformants are plated on media selecting for the cassette and inducing CRISPR/Cas9. Parental p1 plasmids that did not receive the GOI are cut by CRISPR/Cas9, resulting in a cell containing just the desired p1 plasmid. (B) Integration of a model cassette (DNAP1-mUL*) onto p1 in strains with p1-DNAP1-MET15 as the parental plasmid. Agarose gel electrophoresis on DNA extracted from cells after transformation and plating is shown (lanes 2–7). Lane 1 is the parental strain. Biological replicates from transformation without Cas9 induction (lanes 2 and 3) show both parental and desired p1 bands whereas biological replicates from a transformation with the integration cassette and concomitant induction of Cas9 (lanes 4–7) shows only the desired p1 band. (C) Exploration of cassette sizes that can be integrated on p1. Agarose gel electrophoresis on DNA extracted from cells (BY4741 (AJ-Y92)) after transformation of three integration cassettes generating three sizes of p1 – small (14 kb), medium (18 kb), or large (22 kb). Three biological replicates are shown for each size. – pK conditions are controls referring to the lack of proteinase K treatment during DNA preparation; the lack of proteinase K treatment results in the lack of all p1/p2-derived bands because the terminal proteins on p1/p2 prevent migration into agarose gels as previously described.2