Figure 4.
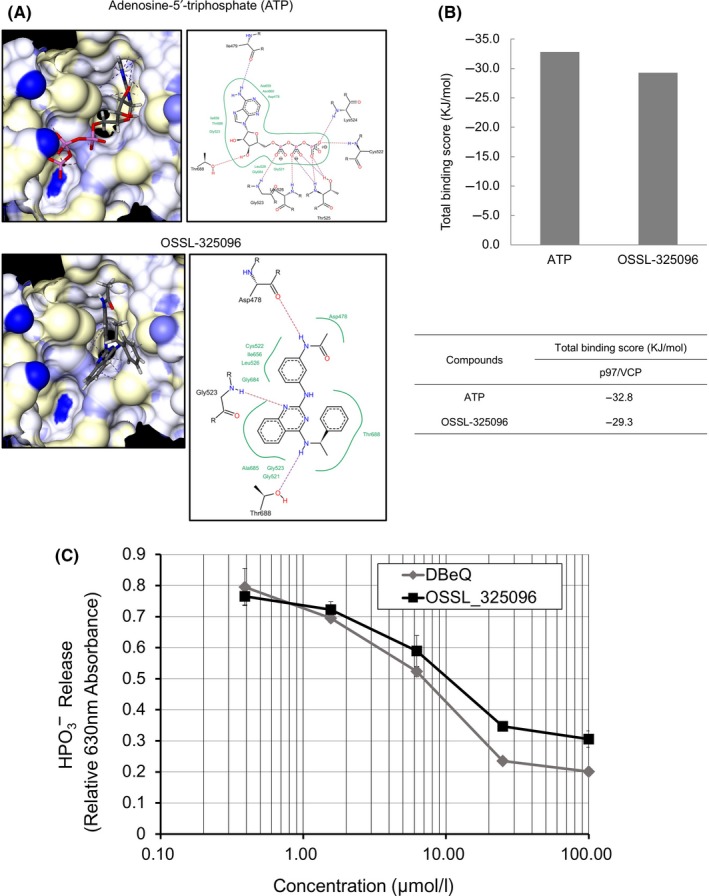
OSSL_325096 functions as an inhibitor of p97/VCP by inhibiting its ATPase activity in vitro. A, Docking simulation results of ATP or OSSL_325096 with the active site of the catalytic domain of p97/VCP (PDB ID: 3CF1). Hydrogen bond interactions between the molecular surface of p97/VCP and compounds are indicated by dotted lines. Hydrophobic interactions are represented as green curves in the 2‐D docking poses. All docking simulations were carried out with LeadIT version 2.1.3 (BioSolveIT GmbH, St Augustin, Germany). B, Summary of the total binding scores between p97/VCP and compounds are shown. Total binding scores were calculated as the sum of receptor‐ligand interactions. C, In vitro ATPase inhibition assay shows that OSSL_325096 suppressed ATPase activity of p97/VCP in a dose‐dependent way. Recombinant p97/VCP was incubated with DMSO or escalating doses of OSSL_325096 or DBeQ before addition of ATP. Degradation of ATP was quantified as HPO 3− release, presented as relative 630 nm absorbance. Data represent mean ± SD derived from three separate experiments
