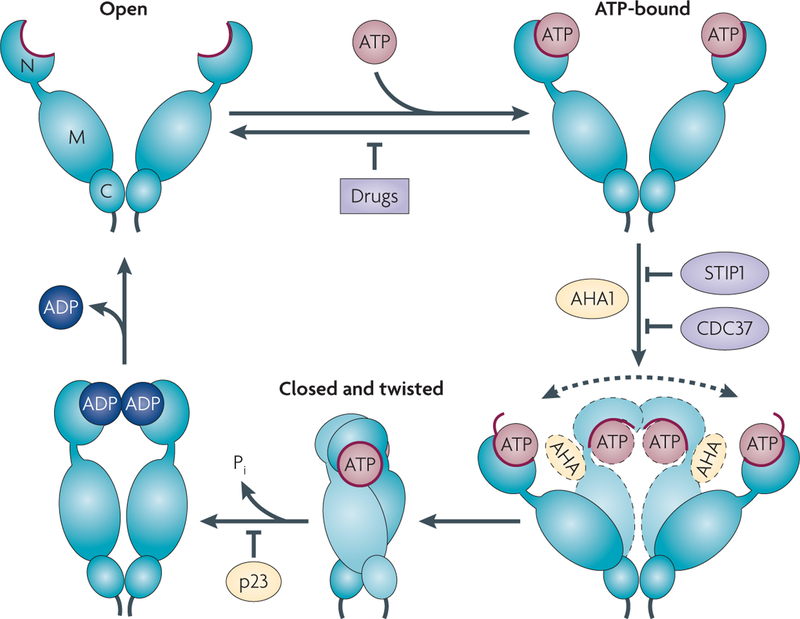
Figure 1 |. The HSP90 chaperone cycle.
Although molecular chaperone heat shock protein 90 (HSP90) samples multiple conformations in the absence of ATP or other factors, current models propose that ATP binding and hydrolysis, as well as a precisely sequenced interaction with an array of co-chaperones, subtly shift the conformational equilibrium, presumably by lowering the energy barrier between certain conformations, thus providing directionality to the HSP90 cycle23,26,27. ATP binding to the undimerized (open) amino terminal (N) domain of HSP90 promotes repositioning of a ‘lid’ segment (red) that leads to transient dimerization of the N domains. Subsequent structural rearrangements result in the ‘closed and twisted’ conformation of HSP90 that is committed to ATP hydrolysis. Binding of the co-chaperone activator of HSP90 ATPase 1 (AHA1) enhances the rate of ATP hydrolysis-dependent HSP90 cycling by increasing the rate of the conformational alterations that result in the acquisition of ATPase competence. The dashed arrow reflects the difficulty of HSP90 in achieving the ATPase-competent conformation in the absence of AHA1. The co-chaperones STIP1 (also known as p60H0P) and cell division cycle 37 homologue (CDC37), and N domain-binding HSP90 inhibitors, exert an opposite effect to that of AHA1 by preventing the initial structural changes necessary for N domain dimerization. Prostaglandin E synthase 3 (PTGES3; also known as p23) slows the ATPase cycle by stabilizing the closed conformation that is committed to ATP hydrolysis. C, carboxy-terminal domain; M, middle domain; P., inorganic phosphate.