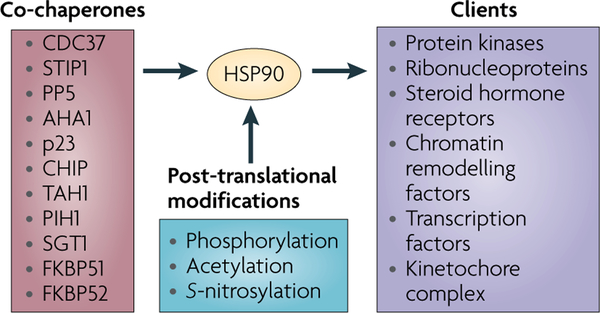
Figure 2 |. Co-chaperones and post-translational modifications modulate HSP90 chaperone activity.
Numerous co-chaperones assist heat shock protein 90 (HSP90) in its chaperone activity. Co-chaperones modulate HSP90 ATPase activity and so determine the rate of chaperone cycling (for example, cell division cycle 37 homologue (CDC37), activator of HSP90 ATPase 1 (AHA1), p23 and STIP1 (also known as p60HOP)), recruit certain classes of client proteins to HSP90, and/or participate in chaperoning specific categories of clients (such as CDC37, STIP1,TAH1, PIH1, suppressor of G2 allele of SKP1 (SUGT1; also known as SGT1), FKBP51 and FKBP52) and post-translationally modify HSP90 itself, its co-chaperones and its client proteins (such as PP5 and CHIP). Post-translational modifications profoundly affect HSP90 function by affecting co-chaperone and client interaction, ATP binding and ATP hydrolysis. The three major regulatory post-translational modifications are phosphorylation, acetylation and S-nitrosylation. An updated list of HSP90-interacting co-chaperones and HSP90-dependent client proteins can be found at the Picard laboratory website (see Further information) maintained by the laboratory of D. Picard, University of Geneva, Switzerland.