Table 1 |.
HSP90 inhibitors in clinical trials
| Structure | Inhibitor | Phase | Route | Source |
|---|---|---|---|---|
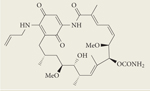 |
Tanespimycin (17-AAG) | II/III | Intravenous | BMS |
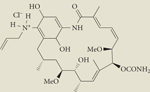 |
Retaspimycin hydrochloride (IPI-504) | II | Intravenous | Infinity |
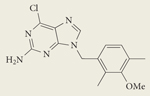 |
BIIB021 CNF2024 | II | Oral | Biogen Idec |
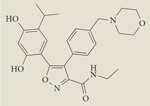 |
AUY922 | I/II | Intravenous | Novartis |
| Resorcinol derivative* | STA-9090 | I/II | Intravenous | Synta |
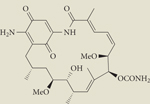 |
IPI-493 | I | Oral | Infinity |
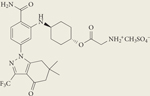 |
SNX-5422 mesylate | I | Oral | Pfizer, Inc. |
| Small molecule* | BIIB028 | I | Intravenous | Biogen Idec |
| Small molecule* | KW-2478 | I | Intravenous | Kyowa Hakko Kirin |
| Small molecule* | AT13387 | I | Oral or intravenous | Astex Therapeutics |
| Small molecule* | XL888 | I | Oral | Exelixis |
| Small molecule* | HSP990 | I | Oral | Novartis |
| Small molecule* | MPC-3100 | I | Oral | Myriad Pharmaceuticals |
| Nanoparticle albumin-bound 17-AAG | ABI-010 | I | Intravenous | Abraxis Bioscience |
HSP90, heat shock protein 90.
The structures are not reported.
