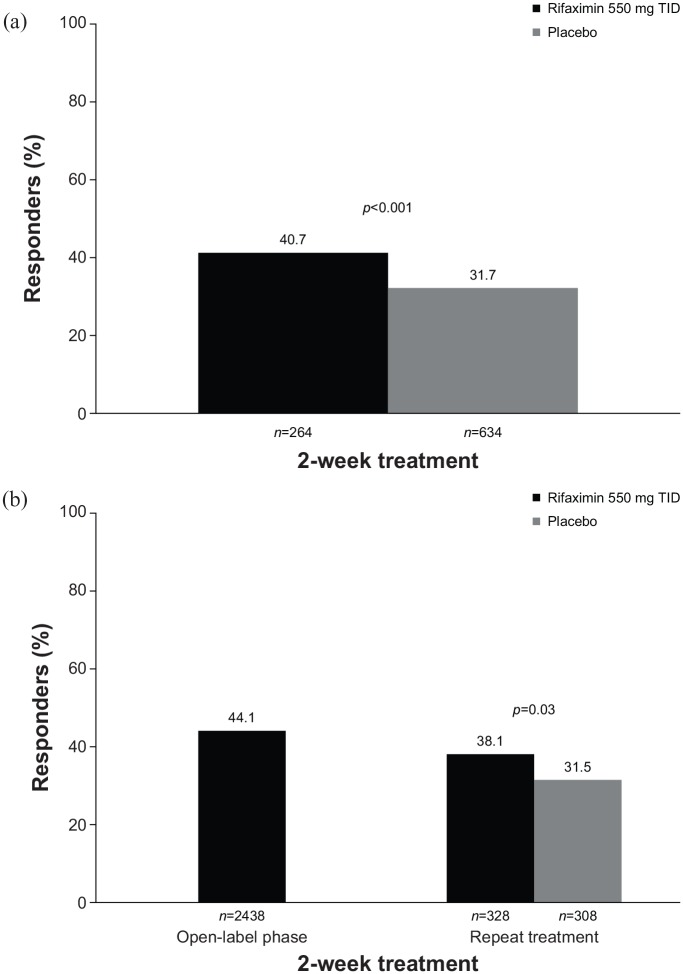
Figure 2.
Percentage of responders to a 2-week course of rifaximin.71,72 (a) Responders were defined as patients with adequate relief of global IBS symptoms (determined by ‘yes’ or ‘no’ response to the weekly question ‘In regard to all your symptoms of IBS, as compared with the way you felt before you started the study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?’) for ⩾2 of the first 4 weeks post-treatment. (b) Responders were defined as patients with ⩾30% decrease from baseline in mean weekly pain score and ⩾50% decrease from baseline in the number of days/week with Bristol Stool Scale type 6 or 7 stool for ⩾2 of the first 4 weeks post-treatment.
IBS, irritable bowel syndrome; TID, three times daily.