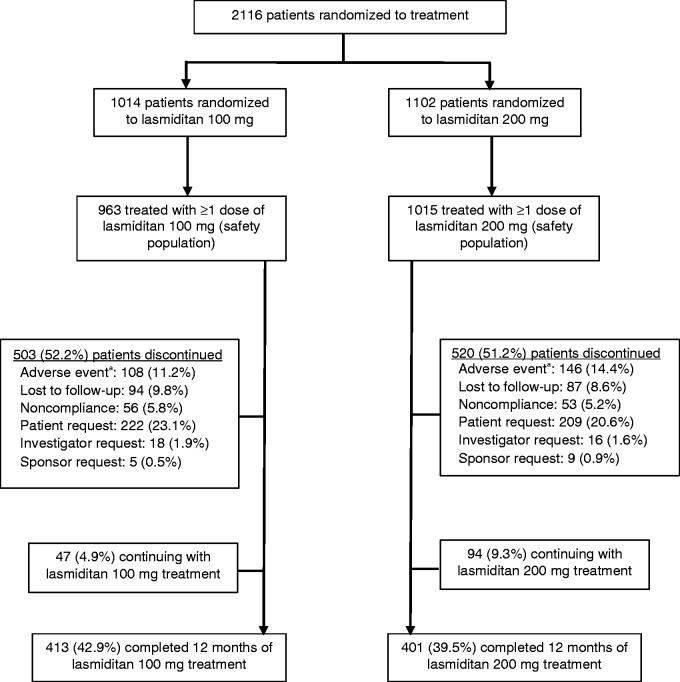
Figure 2.
Flow of study participants.
aIn the safety population, common adverse events leading to discontinuation (>1%) in the lasmiditan 100 mg group included dizziness (26 patients) and somnolence (14 patients) and in the 200 mg group included dizziness (44 patients), paresthesia (19 patients), fatigue (17 patients), nausea (13 patients), and somnolence (11 patients).