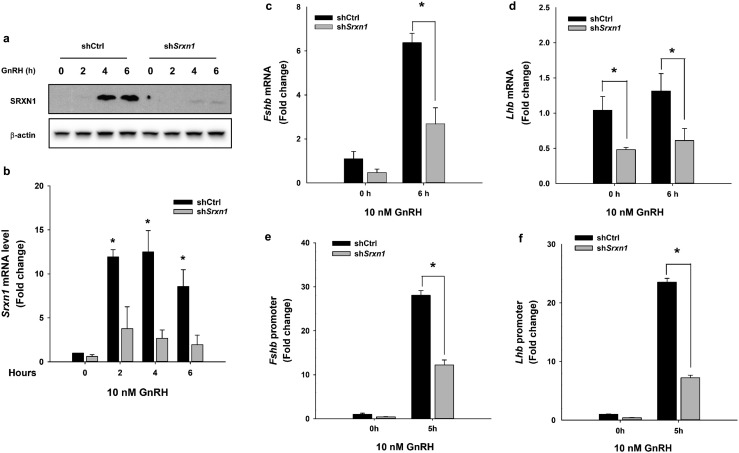
Figure 7.
Knockdown of shSrxn1 in LβT2 cells reduced GnRH-induced gonadotropin gene expression and reporter gene responses. LβT2 cells transduced with lentiviral vectors expressing shRNA for shCtrl or shSrxn1 were generated and selected with puromycin. The serum-starved shCtrl and shSrxn1 cells were treated with or without 10 nM GnRH for the indicated times. Total RNA and protein were harvested and analyzed by western blot or qPCR to evaluate SRXN1 protein and Srxn1 mRNA, respectively. (a) A representative blot from three independent experiments. (b) The chart represents mean Srxn1 values ± SEM normalized to vehicle control from four independent determinations. *P < 0.05 from time zero by Student t test. For analysis of Fshb and Lhb mRNA expression, serum-starved shCtrl and shSrxn1 cell lines were treated with 10 nM GnRH for 6 h. qPCR was performed using specific primers for (c) Fshb, and (d) Lhb. Data shown are the mean ± SEM values from three independent experiments. *P < 0.05, Student t test. Serum-starved shCtrl and shSrxn1 cells were transiently transfected with (e) a −396 bp mouse Fshb promoter or (f) −1.8 kb rat Lhb promoter luciferase reporter plasmid. Transfected cells were treated with 10 nM GnRH for 5 h, the time of maximal reporter gene response. Luciferase values were internally normalized using a cotransfected β-galactosidase reporter to control for transfection efficiency. Data are reported as mean ± SEM from three independent experiments. *P < 0.05, by Student t test.