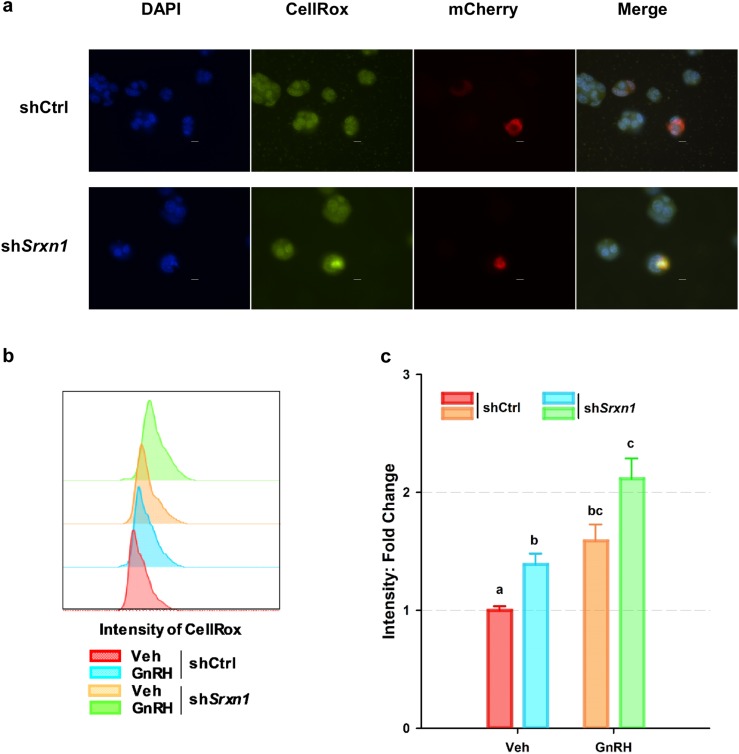
Figure 8.
Knockdown of shSrxn1 enhanced ROS production in LβT2 cells. LβT2 cells were transiently transfected with shCtrl or shSrxn1 expression vectors and pLV-mCherry to mark transfected cells (red). At 48 h posttransfection, cells were serum starved and treated with 10 nM GnRH for 4 h and then incubated with CellRox (green) to detect the presence of ROS. Cells were fixed with 4% formaldehyde, and nuclei were stained with DAPI (blue) to illuminate nuclei. (a) Representative micrographs are shown. The shCtrl and shSrxn1 cell lines were treated with or without GnRH for 4 h and given a 1-h rest to allow induction of SRXN1 expression. After resting, cells were retreated with a single pulse of vehicle or GnRH for 30 min and changed to normal serum-free medium and incubated an additional 30 min. Cells were incubated with CellRox for 15 min followed by 4% formaldehyde fixation for 20 min. Scale bar, 10 µm. (b) Cells were evaluated by flow cytometry, and fluorescein isothiocyanate–A frequency distribution of intensity was compared between shCtrl and shSrxn1 cells with or without GnRH. (c) Geometric means of fluorescein isothiocyanate–A signals for CellRox were extracted and normalized to the ratio of shCtrl cells treated with vehicle. Error bars denote SEM from at least four set samples in each. Means were compared using two-factor ANOVA and post hoc testing with Dunnett comparison with control test using ShCtrl cells treated with vehicle (Veh) as the control. Groups with different letters are significantly different (P < 0.05).