Abstract
Neuropathic pain in pediatric oncology results from distinct causes of lesions or disease processes affecting the somatosensory system including chemotherapy-related neuronal injury, solid tumor related involvement of neural structures, post-surgical neuropathic pain including phantom limb pain and post-limb sparing pain, and the complex circumstances of neuropathic pain at the end of life. While treatment algorithms reflect the general treatment principles applied for adult neuropathic pain, the dose regimens applied in children are modest and rarely escalated to the maximum doses to optimize analgesic efficacy. Pharmacological management of neuropathic pain should be based on a stepwise intervention strategy, as the most effective approach is with combinations of medications. Gabapentinoids and tricyclic antidepressants are recommended as first line therapy agents. Methadone, ketamine, and lidocaine may be useful adjuvants in selected patients.
Prospective studies extended over a substantial length of time are recommended based on the nature of neuropathic pain as persistent, chronic pain and based on the need for sufficient time to escalate medication dose regimens to full analgesic efficacy.
1. Background
Neuropathic pain (NP) is defined by the International Association for the Study of Pain (IASP) Special Interest Group on Neuropathic Pain (NeuPSIG) as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” [1]. Successful treatment of NP conditions is challenging and may require multiple lines of therapy; pain may be difficult to adequately control leading to the concept of “pharmaco-resistant NP” [2]. While NP is well characterized in adults with cancer [3–15], it is less well described in pediatric oncology [16–22]. In the setting of pain in pediatric oncology patients, NP can be caused by a variety of types of somatosensory system injuries including chemotherapy-related NP [19, 23], tumor-related NP [24–26], post-surgical NP [27, 28], and the complex circumstances of NP at the end-of-life [29]. The most commonly incriminated chemotherapy agent related to neurotoxicity and NP in children is vincristine. Vincristine-related NP has been reported in children with acute lymphoblastic leukemia [23], as a dose-dependent effect mediated by inhibition of microtubules polymerization and disruption of neuronal axons [30]. Pediatric solid tumors in the proximity of nerve structures can directly cause tumor-related NP [24–26]. Post-surgical NP related to mechanical nerve trauma in pediatric oncology has a prevalence of 81.6% after surgery for osteosarcoma with either post-amputation phantom limb pain (PLP) or as NP after limb sparing surgery [27]. NP at the end of life is often related to complex circumstances and may include mixed nociceptive and neuropathic mechanisms [29].
2. Assessment of NP in Children
Difficulties in assessment of NP in children may include age-related developmental limitations in the ability to differentiate NP descriptors (i.e. “burning”, “tingling”, “needles and pins”, “shooting”, “lancinating”), from nociceptive pain descriptors (i.e. “aching”, “pressure”, and “dull pain”), and to communicate the specific characteristics of pain [31]. There are no validated scales for NP for children under the age of 5 years. Quantitative sensory testing is effective to diagnose NP; nevertheless, it requires expensive equipment and trained personnel, which may limit its use outside of research settings [32]. NP specific clinical assessment tools have been developed for children with cancer [19, 33]. The pediatric modified Total Neuropathy Scale (ped-mTNS) was the first reported screening tool for pediatric chemotherapy induced peripheral neuropathy, validated for children ages 5–18 years [33]. Ped-mTNS includes interview questions related to sensory, motor, and autonomic symptoms, and physical findings related to light touch sensation, pin sensation, vibration exam, strength, and deep tendon reflexes [33]. A variation of this assessment tool was developed for children experiencing vincristine-related NP, the Total Neuropathy Score Pediatric Vincristine (TNS-PV). This modified version of the ped-mTNS is based on the addition of constipation and hoarseness to the evaluation tool and is valid for children ages 6–18 years [19]. A summary of screening and assessment tools for NP in children is presented in Table 1. The peds-mTNS appears to be the most valid tool for NP screening purposes in pediatric oncology [34].
Table 1.
Assessment Tools for Pediatric Neuropathic Pain
| Tool | Proposed Target Population | Advantages/Disadvantages | ||
|---|---|---|---|---|
| I. Screening Tools | ||||
| 1. Pediatric-modified Total Neuropathy Score 3 sets of symptom questions 5 physical examination signs |
Children aged 5–18 years with chemotherapy-induced peripheral neuropathy | Good reliability and internal consistency Good for screening and assessment Not as sensitive to change over time Clinical significance of scores is unclear |
||
| II. Assessment Tools | ||||
| 2. Adolescent Pediatric Pain Tool Body outline Word graphic rating scale List of 67 symptom descriptors |
Children aged 8–17 years with acute pain (hospitalized or not) | Good validity, reliable in a variety of pain conditions Quickly completed Multidimensional approach to pain Not well studied in chronic or complex pain |
||
| 3. Pediatric Pain Questionnaire 10-cm Visual Analog Scale 46 symptom descriptors Pain location |
Children aged 5–18 years Primarily used in patients with juvenile rheumatoid arthritis and sickle-cell anemia | Derived from the well-studied McGill Pain Questionnaire Well-established evidence of validity Takes 15 min to complete |
||
| 4. Abu-Saad Pediatric Pain Assessment Tool 10-cm Visual Analog Scale 32 symptom descriptors |
Children aged 5–17 years with disease-related pain or undergoing surgery | Assesses multiple aspects of pain, including triggers and medication type/amount Good internal consistency Less supportive evidence than for other tools |
||
Reproduced with permission from [34] - The assessment tools were validated for pain in children; they may be helpful for NP because they assess the pain characteristics
3. Treatment Concepts for NP in Adults
Given the difficulties faced by clinicians in achieving successful treatment of NP in general and the fact that the current evidence shows limited efficacy with high numbers needed to treat (NNT) in adult randomized controlled studies (RCTs), the approach to treatment of NP in adults has been based on the development of evidence-based consensus guidelines from various groups of experts [15, 35–37]. The need for such guidelines emerged from several limitations of the existing literature including: 1) the fact that the current clinical practice is mostly driven by evidence based on RCTs on the clinical entities of post-herpetic neuralgia and diabetic peripheral neuropathy which are most prevalent in the adult population; 2) the limited evidence from comparison trials of various treatment interventions; 3) the inconsistency of pain outcome measures among studies; and 4) the limited duration of studies, often shorter than the persistent chronic nature of most NP entities [15].
Literature is consistent regarding the recommendation that the pharmacological management of NP should be based on a stepwise intervention strategy. Historically, the sequence of pharmacological interventions for NP has consisted of tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) as first line of treatment [15, 38]. Subsequently, α2δ agonists (gabapentin and pregabalin) have been added as first line interventions [39–41]. Tramadol and strong mu opioid receptor agonists have represented second line therapy options [15, 38–41], and more recently, topical medications have been added to the second and third line of therapy including 5% lidocaine patch, 8% capsaicin patch, and subcutaneous injection of Botulinum toxin type A (Supplementary material) [39, 41]. In general, opioids are recommended late in the escalation algorithm to follow all of the other options of treatment [15, 38–41]. Considerations in the indication for opioids later in the strategy algorithm may include the risks of opioid related side effects as well as development of opioid tolerance, opioid hyperalgesia and dependence. Dosing for NP therapies in adults is well established (Supplementary material).
One important consideration in the algorithm of clinical decision-making and opioid selection for the pediatric cancer patient regards the specific effects of opioids on the immune system. These effects may have clinical implications for cancer outcomes including cancer progression, recurrence, disease-free survival, and metastases. In making these clinical decisions, one has to remember the lack of evidence in the literature reflective of pediatric cancer patients, as our knowledge of immune function implications of opioids comes from experimental data and adult clinical studies.
The most effective way to treat NP is with combinations of medications [11, 15]. The need for combination therapy may be partially explained by the limited efficacy of each individual class of drugs as monotherapy, which is supported by the high NNT for most of the NP medications (Supplementary material) [39].
Cancer-related NP is challenging to treat because patients often experience multiple sources of pain from disease and/or treatment, leading to high opioid exposure in this population [11]. While lines of therapy for cancer related NP in adults follow the general strategies used for non-cancer related NP, starting with TCAs, SNRIs, and gabapentinoids as first line therapies, the dose regimens seem to have not been escalated to the maximum doses reported in the general NP literature [4, 5, 7–10].
4. Limitations of Understanding and Treating NP
Several contributory factors may lead to difficulties in understanding the various NP entities and developing effective treatment strategies. Patients with NP represent a heterogeneous population and the mechanisms of generating NP are distinct and variable. In oncology, NP can be conceptualized as distinct categories with different pathophysiology: chemotherapy-related NP [19, 23], tumor-related NP [24, 25], post-surgical NP [27, 28], and the complex circumstances of NP at the end-of-life [29]. In the medical practice in general, there are multiple causes and mechanisms of NP; therefore, approaching NP based on specific pathophysiologic mechanisms may be preferable to the generalization of NP as a single entity. The nature of NP as persistent chronic pain for extended period of time may be in conflict with the fact that most of the literature reports pharmacological treatment trials for short durations, on average 1–2 months, which may limit the understanding of outcomes. Lastly, pain outcomes may be measured in various ways, and the point at which the therapy is considered efficacious may vary across studies, which may contribute to difficulties in comparing the efficacy of different therapeutic interventions for NP across literature.
5. NP in Pediatric Oncology – Evidence for Pharmacological Treatment
The literature describing NP in pediatric oncology is mostly represented by case reports and case series, while stronger evidence from prospective RCT or retrospective studies is limited. Limiting factors to advancing clinical research may include difficulties differentiating descriptors of pain leading to misdiagnosis or delayed diagnosis of NP in children and the overall small population of children with NP.
We searched the PubMed and Web of Science databases from 2000 to 2018 using the following terms: (neuropath*[Title]) AND pain [Title] AND (pediatric OR paediatric OR child OR childhood OR adolesc*) and we reviewed the evidence relevant to pediatric NP in general and pediatric NP in children with cancer. Based on this search method, the literature pertinent to NP in children with cancer is limited to cumulative reports of 235 children [21, 24, 25, 42–46], while the evidence for the general pediatric population with NP is generated from a cumulative number of 376 patients [47–51]. Cumulative reports of mild-to-moderate adverse effects of lines of pharmacological therapy for NP in children include 23 children, of which 10 are pediatric oncology patients [47–51].
The pediatric patient population details, the category of NP, and the medication dosing regimen, efficacy, adverse reactions and duration of treatment are presented in Table 2. For comparison, Table 3 reflects similar data pertaining to the literature evidence of pharmacological treatment of NP in the general pediatric population. Most of the pediatric oncology literature pertinent to the treatment of NP describes treatment options for chemotherapy related NP (cumulative n = 187), including 2 small size case series reporting the use of methadone [21, 43], a retrospective review of gabapentin use for vincristine-induced NP [23], and one open label trial of pregabalin [42] (Table 2). Treatment of tumor-related NP is reported in 2 case reports or series (cumulative n = 5), both regarding the use of gabapentin.
Table 2.
Medications Regimens for Neuropathic Pain in Pediatric Oncology
| Reference | Design and Patients Data: age, diagnostic, type of pain | Main Neuropathic Pain Medication (Additional Neuropathic Pain Medications) |
Analgesic Efficacy Measures | Adverse effects | Duration |
|---|---|---|---|---|---|
| Chemotherapy-related NP | |||||
| Anghelescu 2011 [23] n = 153 |
Retrospective: ALL, vincristine related NP, median (range) age 7.9 (1.3–19.2) years |
Gabapentin: 62.2% of NP episodes treated with gabapentin, starting mean dose 14.2 mg/kg, prophylactic mean dose 15.8 mg/kg, recurrent NP mean dose 18.1 mg/kg (37.8% of episodes treated with opioids) |
Efficacy measures not evaluated | Adverse effects not evaluated | Duration not evaluated |
| Vondracek 2009 [42] n = 30 |
Prospective: solid tumor or leukemia, chemotherapy-related NP, mean (range) 13.5 (10–17) years | Pregabalin initiated at 75 mg/day, escalated to 150–300 mg/day (5 mg/kg) | Mean VAS decreased by 59% | 4 patients: sleepiness, nausea, vomiting, drowsiness, dry mouth | 8 weeks |
| Madden 2017 [21] n = 2 |
19 y/o, ALL, vincristine-related NP |
Methadone PO 0.03 mg/kg/dose (amitriptyline 0.25 mg/kg, gabapentin 45 mg/kg, olanzeapine 0.5 mg) |
pain scores, fatigue, and insomnia decreased | No adverse effects reported | 1 year+ |
| 6 y/o, ALL, vincristine-related NP |
Methadone PO 0.04 mg/kg/dose (gabapentin 45 mg/kg, morphine 0.2 mg/kg/dose) |
pain scores, fatigue, and insomnia decreased, morphine use decreased | No adverse effects reported | Not reported | |
| Rasmussen 2015 [43] n = 2 |
11 y/o, pre-B ALL, vincristine-related NP |
Methadone IV 21.8 mg/kg/day Methadone IV PCA 12.7 mg/kg/day (nortriptyline, amitriptyline, pregabalin) |
NRS decreased 4 points | Drowsiness, withdrawal | 409 days |
| 17 y/o relapsed metastatic Ewing’s sarcoma, vincristine-related NP |
Methadone IV 4.8 mg/kg/day Methadone IV PCA 20.0 mg/kg/day (nortriptyline, amitriptyline, gabapentin, oxycarbazepine) |
NRS decreased 5 points | Hypotension, CNS depression | 207 days | |
| Tumor-related NP | |||||
| Butkovic 2006 [24] n = 4/5 |
18 y/o, metastatic osteosarcoma, somatic, visceral, NP |
Gabapentin 10, 20, 30 mg/kg/day for 3 consecutive days, respectively, 4 weeks at maximum dose, maintenance dose 10 mg/kg/day (morphine, methadone, fentanyl patch, amitriptyline, NSAIDs, benzodiazepine) |
VAS decreased from 10 to 3–4 | Sedation and fatigue | 1 week** |
| 17 y/o, pelvis Ewing’s sarcoma, NP |
Gabapentin started at 30 mg/kg/day, 3 weeks at maximum dose, maintenance dose 10 mg/kg/day (amitriptyline, tramadol, fentanyl PCA, transdermal fentanyl, NSAIDs) |
VAS decreased from 10 to 4; fentanyl dose decreased | Dizziness | 10 days** | |
| 14 y/o, metastatic ovarian carcinoma, NP |
Gabapentin titrated up to 30 mg/kg/day for 2 weeks; maintenance dose 15 mg/kg/day (amitriptyline, transdermal fentanyl, ibuprofen) |
VAS decreased from 9 to 3, amitriptyline dose decreased | No adverse effects reported | 10 days** | |
| 15 y/o, osteosarcoma, NP |
Gabapentin titrated up to 25 mg/kg/day for 2 weeks, maintenance dose 20 mg/kg/day (tramadol, acetaminophen) |
VAS decreased from 8 to 4 | No adverse effects reported | 5 days** | |
| Keskinbora 2004 [25] n = 1 |
12 y/o, Ewing’s sarcoma, NP | Gabapentin: 100, 200, 300 mg/day for 3 consecutive days, followed by 300 mg TID | Burning and lancinating pain VAS decreased from 8 to 0 | No adverse effects reported | 1 month |
| Surgery-related NP | |||||
| Anghelescu 2017 [27] n = 30 |
Prospective: osteosarcoma, NP after limb sparing or amputation, median age 13.3 years |
Gabapentin: 26 patients - 20.2 (43.8) mg/kg/day 5 patients also treated with amitriptyline 0.5 (0.7) mg/kg/day 4 patients also treated with methadone 0.3 mg/kg/day* (4 patients treated with opioids only) |
Treatment continued until disappearance of NP symptoms | Adverse effects not evaluated | Mean (SD) 9.5 (8.1) weeks |
| EOL-related NP | |||||
| Kajiume 2012 [44] n = 1 |
5 y/o, relapsed T-cell lymphoma, NP, EOL |
Lidocaine 9.3–14 mcg/kg/min ketamine 0.12 mg/kg/h (dexamethasone, fentanyl) |
Full relief of pain and return to normalcy | No adverse effects reported | 47 days |
| Klpestad 2001 [45] n = 1 |
12 y/o, glioblastoma multiforme, tumor-induced NP, EOL |
Ketamine for 4 weeks, 7.5 to 10 mg bolus dose, followed by continuous IV infusion, increased continuously until death (morphine) |
Allowed patient to live at home for EOL care | Myoclonic movements | 67 days |
Values reported as: starting doses (maximum doses)
Duration reflects when analgesic efficacy was reached
Abbreviations: NP, neuropathic pain; VAS, visual analog score (0–10); NSAIDs, nonsteroidal anti-inflammatory drugs; PCA, patient-controlled analgesia; ALL, acute lymphoblastic leukemia, NRS, numeric pain score; PLP, phantom limb pain; EOL, end of life
Table 3.
Medications Regimens for Neuropathic Pain in General Pediatrics
| Reference | Design and Patients Data: age, diagnostic, type of pain | Main Neuropathic Pain Medication (Additional Neuropathic Pain Medications) |
Analgesic Efficacy Measures | Adverse effects | Duration |
|---|---|---|---|---|---|
| Akkurt 2015 [47] n = 1 |
12 y/o, NP, sciatic nerve injury | Gabapentin 10 mg/kg/day for 2 weeks, followed by 16 mg/kg/day | Considerable improvement of NP symptoms | Light dizziness for the first 2 days of therapy | 2 months |
| Brown 2016 [48] n = 34 |
Randomized control trial: 34 patients, CRPS I or NP, mean (SD) age: amitriptyline group: 13.5 (2.35) gabapentin group: 12.6 (2.52) |
Amitriptyline 10 mg/day, Gabapentin Day 1–3: 300 mg Day 4–6: 300 mg BID Day 7- end: 300 mg TID |
Decrease in CAS score for amitriptyline = 1.50 Decrease in CAS score for gabapentin = 1.77 Sleep scores improved for both |
3 patients: additional pain sites | 6 weeks |
| Butkovic 2006 [24] n = 1/5 |
5 y/o, idiopathic NP |
Gabapentin 10, 20, 30 mg/kg/day for 3 consecutive days, respectively, 2 weeks at maximum dose, maintenance dose 10 mg/kg/day (ibuprofen, amitriptyline)* |
VAS decreased from 9 to 3 | Sedation and fatigue | 1 week |
| Orellana Silva 2013 [49] n = 12 |
Prospective: 12 patients, NP from burn sequelae, mean (SD) age 11 years 7 months (2 years 6 months) | 5% lidocaine patch, 12 hour on, 12 hour off | Mean FACES score decreased from 7 to 0 Mean DN4 score decreased from 6 to 2 |
No adverse effects reported | 3 months |
| Nayak 2008 [54] n = 5 |
12 y/o, surgical scar-related NP post-nephrectomy | One 5% lidocaine patches for 12 hrs/day for 1 month | VAS decreased from 7 to 2 | No adverse effects reported | 3 months |
| 15 y/o, imperforate anus, NP at colostomy site |
Two 5% lidocaine patches for 12 hrs/day for 1 week (gabapentin, amitriptyline)* |
No improvement | No adverse effects related to patch | 1 week | |
| 18 y/o, sacrococcygeal teratoma, scar-related NP post-thoracotomy |
Two 5% lidocaine patch for 12 hrs/day for 3 months (codeine, NSAIDs, paracetamol, gabapentin)* |
VAS decreased from 9 to 0 | No adverse effects reported | 3 months | |
| 11 y/o, congenital heart disease, scar-related NP at femoral catheter entry site |
One 5% lidocaine patch for 12 hrs/day for 3 months (paracetamol, NSAIDs, amitriptyline 10 mg)* |
VAS decreased from 8 to 4 at 3 months and to 3 at 6 months | No adverse effects reported | 4 months | |
| 14 y/o, scar-related NP post laminoplasty | Two 5% lidocaine patches for 12 hrs a day for 3 months | VAS decreased from 8 to 0 at 1 month | No adverse effects reported | 3 months | |
| Taylor 2015 [50] n =14 |
Patients with NP, median (range) age 15.5 (1 month - 23 years) |
Ketamine IV 0.05 to 0.1 mg/kg increased in increments of 0.05 mg/kg every 5 to 10 mins, maximum 0.6 mg/kg (morphine PCA - measure of pain)* |
Decrease in opioid use and decrease in pain | 3 patients, fatigue, imbalance, disorientation, shakiness, slurred speech, agitation, myoclonic jerks, and hemorrhagic cystitis | 7 days (discharged on ketamine) |
| Yazde Puleio 2018 [51] n = 74 |
Patients with various types of NP, 59 cancer, 13 other, mean (SD) age 10.9 (5.36) years | Opioid rotation, morphine as initial opioid in 57 cases, rotation required in 35 patients, most frequent switch - morphine to methadone | Better quality of life | 6 patients had adverse effects (not specified) |
Concurrent medications are included as mentioned in the primary source
Abbreviations: NP, neuropathic pain; CRPS, chronic regional pain syndrome; CAS, to check; VAS, visual analog score (0–10); NSAIDs, nonsteroidal anti-inflammatory drugs; PCA, patient-controlled analgesia
The incidence and treatment options for post-surgical NP caused by mechanical trauma, either by nerve stretching and pulling, as in the limb sparing surgery, or by severing the nerves, as in the amputation surgery have been reported [27, 28, 46, 52]. The generally accepted first lines of pharmacological interventions for NP are applied, including gabapentinoids and TCAs [27, 28, 52]. Among NP medications, gabapentin started preoperatively has been reported as preemptive treatment of NP [27, 28]. In the setting of chronic post-surgical pain, which has a neuropathic component, the use of neuraxial (epidural) analgesia and continuous peripheral nerve blocks has been advocated [27, 28].
NP related to pediatric cancer at the end of life can pose therapeutic challenges due to complex multiple pain generators and/or generalized pain; therefore, multimodal NP therapy including high dose opioids may be required to control pain and improve quality of life [29]. Clinicians may also consider the options of neuraxial or peripheral nerve blocks, which can contribute to reducing the opioid exposure and improve pain control at the end of life [53]. Successful treatment of NP at the end of life may necessitate successive or concurrent lines of therapy, including gabapentin, TCAs, methadone, ketamine and/or lidocaine infusions (Table 2).
a. Gabapentinoids
Based on the literature describing the use of gabapentin for NP in children (cumulative n = 210), the dosing regimen starts at 10 mg/kg/day and escalates to 30 mg/kg/day over a few days to a week [23–25]. The highest doses reported, in a case report and in a prospective study of post-surgical NP were 45 and 43.8 mg/kg/day, respectively [21, 27] (Table 2 and 4). The literature in general reflects under-dosing strategies in children, compared to the adult dosing recommendation to escalate up to 3600 mg/day, or 50–70 mg/kg/day. While the pediatric literature reviewed here suggests that analgesic efficacy was achieved in the dose ranges reported, one should consider that most reports reflect concurrent use of several lines of NP medications which may explain attaining analgesic efficacy at lower dose ranges.
Table 4.
Summary of Dose Regimens Reported for Neuropathic Pain in Pediatric Patients
| Medication and Cumulative Number of Patients | Dose Regimen (daily doses)* | Summary and Recommendation for Clinical Practice |
|---|---|---|
|
Gabapentin n = 210 |
Mean starting dose: 20.2 mg/kg Maximum dose: 43.8 mg/kg (Anghelescu 2017 [27]) |
Titrate up to 30 mg/kg/day |
|
Mean starting dose 14.2 mg/kg (Anghelescu 2011 [23]) | ||
|
10 mg/kg for 2 weeks, increased to 16 mg/kg (Akkurt 2015 [47]) | ||
|
Days 1–3, 4–6 and 7 and up: 300 mg (~6 mg/kg)
**, 300 mg BID (~12 mg/kg)**, and 300 mg TID (~18 mg/kg)**, respectively (Brown 2016 [48]) | ||
|
Day 1, 2 and 3: 10, 20 and 30 mg/kg, respectively 2–4 weeks at maximum dose, maintenance 10 mg/kg (Butkovic 2006 [24]) | ||
|
30 mg/kg for 3 weeks, maintenance 10 mg/kg (Butkovic 2006 [24]) | ||
|
Dose titrated up to 30 mg/kg for 2 weeks, maintenance 15 mg/kg (Butkovic 2006 [24]) | ||
|
Dose titrated up to 25 mg/kg for 2 weeks, maintenance 20 mg/kg (Butkovic 2006 [24]) | ||
|
Day 1, 2, 3: 100 mg (~2.9 mg/kg)
**, 100 mg BID (~5.7 mg/kg)**, 100 mg TID (~8.6 mg/kg)**, then 300 mg TID (~25.7 mg/kg)** (Keskinbora 2004 [25]) | ||
|
45 mg/kg (Madden 2017 [21]) | ||
|
500 mg (~8.9 mg/kg)** (Nayak 2008 [54]) | ||
|
9 mg/kg BID (Rasmussen 2015 [43]) | ||
|
Pregabalin n = 31 |
0.8 mg/kg TID (Rasmussen 2015 [43]) |
Titrate up to 5 mg/kg/day |
|
75 mg escalated to 150–300 mg (5 mg/kg) (Vondracek 2009 [42]) | ||
|
Amitriptyline n = 58 |
0.5–0.7 mg/kg (Anghelescu 2017 [27]) |
0.35–0.4 mg/kg/day |
|
10 mg (Brown 2016 [48]) | ||
|
10 mg (Butkovic 2006 [24]) | ||
|
10 mg BID (Butkovic 2006 [24]) | ||
|
20 mg BID (Butkovic 2006 [24]) | ||
|
2.5 mg (0.25 mg/kg) (Madden 2017 [21]) | ||
|
10 mg QD (~0.37 mg/kg)** (Nayak 2008 [54]) | ||
|
0.35 mg/kg (~20.0 mg)** (Rasmussen 2015 [43]) | ||
|
0.9 mg/kg (~55.8 mg)** (Rasmussen 2015 [43]) | ||
|
Nortriptyline n = 2 |
0.35 mg/kg (~20.0 mg)**
BID (Rasmussen 2015 [43]) |
0.35–0.4 mg/kg/day |
|
0.4 mg/kg (~24.8 mg)**
TID (Rasmussen 2015 [43]) | ||
|
Methadone n = 34 |
0.3 mg/kg PO (Anghelescu 2017 [27]) |
0.06–32.7 mg/kg/day |
|
0.03 mg/kg/dose (0.3 mg BID) PO (Madden 2017 [21]) | ||
|
0.04 mg/kg/dose (1 mg BID) PO (Madden 2017 [21]) | ||
|
32.7 mg/kg/day IV (Rasmussen 2015 [43]) | ||
|
24.8 mg/kg/day IV (Rasmussen 2015 [43]) | ||
|
Ketamine n = 16 |
0.12 mg/kg/hr IV (Kajiume 2012 [44]) |
0.05–0.6 mg/kg/hr |
|
7.5–10 mg bolus, continuous infusion (~0.19–0.40 mg/kg)** (Klpestad 2001 [45]) | ||
|
0.05–0.1 mg/kg, increased by 0.05 mg/kg every 5–10 min, maximum 0.6 mg/kg/hr (Taylor 2015 [50]) | ||
|
Lidocaine n = 18 |
9.3–14 mcg/kg/min (Kajiume 2012 [44]) |
IV: 14 mcg/kg/min (0.84 mg/kg/hr) Patch: 5% patch Q12hrs |
|
5% lidocaine patches, Q12 hrs (Nayak 2008 [54]) | ||
|
5% lidocaine patches, Q12 hrs (Orellana Silva 2013 [49]) |
Doses in mg/kg/day unless otherwise specified;
Values estimated based on weight
The comparison between gabapentin dose regimens reported in two pediatric oncology studies, one retrospective study of NP related to vincristine during treatment for ALL and one prospective study of post-surgical NP following limb sparing and amputation, leads to the observation that post-surgical NP is treated with higher dose regimens than chemotherapy-related NP. While the mean dose of gabapentin for vincristine-related NP varied from 18.1 mg/kg/day [SD 7.6] to 15.8 mg/kg/day [SD 8.3] in groups of patients treated for existing NP versus prophylactically [23], the patients treated for post-surgical NP received gabapentin starting at 20 mg/kg/day and escalated up to 43.8 mg/kg/day [27] (Table 2 and 4). For post-surgical NP, 20 of 37 patients received gabapentin initiated pre-operatively and the majority (65.4%) received gabapentin as a single NP therapy.
Pregabalin use is reported in one open label pilot trial of 30 pediatric oncology patients with solid tumor or leukemia, as a regimen of 75 mg/day escalated to 150–300 mg/day (5 mg/day) [42] (Table 2 and 4). Compared to adult dosing regimens (8.5–12 mg/kg), like gabapentin, pregabalin appears to be under-dosed in children. When reported in association with other NP medications, pregabalin is administered at lower doses (2.4 mg/kg/day) [43]. Gabapentin and pregabalin have a favorable side effect profile and are well tolerated. Although dizziness and sedation may occur, they are dose-dependent and can be avoided by initiation of therapy at a low dose and slow up titration. Dosage reduction is necessary in circumstances of renal insufficiency, based on creatinine clearance [15].
b. Tricyclic antidepressants
Amitriptyline [21, 24, 43, 48, 54] and nortriptyline [43] are citied in pediatric NP algorithms in the dosing range of 0.35–0.4 mg/kg/day (Table 4). Doses between 10 mg/day to 20 mg BID have also been reported. Adult literature reflects dosing regimens of 0.35–3 mg/kg/day, which is an indication that the maximum dose regimens are not reached in children. Within this dose range, doses used for the indication of chronic pain are lower than those needed to achieve the antidepressant effect. Dose escalation may be limited by side effects including anticholinergic effects, like dry mouth, orthostatic hypotension, constipation, and urinary retention, as well as cardiac conduction abnormalities including prolonged QT interval, and risk of torsade de pointes and sudden cardiac death [15]. It is customary in our practice to obtain a baseline EKG before initiation of a tricyclic antidepressant regimen and to consider carefully the possible concurrent medications with QT prolonging effect and prioritize their use based on risk benefit ratios in each clinical situation.
c. Methadone
In a retrospective study of 41 pediatric patients (mean age 15.7 years [range 0.6–23 years]) with oncologic or hematologic diagnoses treated with methadone [55], the indications included: nociceptive pain unresponsive to opioids (n=17 [33%]), neuropathic pain (n=20 [39.2%]), facilitation of opioid weaning (n=11 [21.6%]), and end-of-life pain management (n=3 [5.9%]). The median (range) starting dose was 0.32 (0.06–3.8) mg/kg/day and the highest dose was 9.4 mg/kg/day. The enteral route was preferred, with only 3 patients receiving IV methadone. Analgesic efficacy was reached in 52.9% and 40% of patients with nociceptive pain and NP, respectively. Sedation was the most common side effect, and no respiratory depression was noted. In general, the literature suggests the same dosing for methadone as described above, except the case series of two patients who were prescribed continuous IV methadone and PCA methadone [43] (Table 4). Both patients in this report were previously treated with strong opioids resulting in opioid tolerance and explaining the need for higher doses of methadone. The risk benefit considerations when initiating therapy with methadone include the risk of prolonged cardiac conduction, with QT prolongation and risk of torsade de points. Concurrent risk factors and medications with QT prolongation effects should be evaluated. In our institution, therapy with methadone for pediatric chronic pain is limited to consultation with a pain medicine specialist and/or palliative care physician.
d. Ketamine
The use of ketamine for NP in pediatric oncology has been reported in the context of end of life, starting as low dose regimens, with dose escalation as needed, either as bolus doses or as infusions [44, 45, 50]. Reported dose regimens were initiated at 0.05 mg/kg and escalated to a maximum dose of 0.6 mg/kg/hr (Table 4). In our pediatric oncology pain practice, ketamine infusions are used for clinical circumstances of intractable NP unresponsive to standard lines of therapy or when the escalation of treatment algorithms was prevented by contraindications or side effects to the standard lines of therapy. Continuous infusion doses used in our practice are 0.025 mg-0.3 mg/kg/hr. Required monitoring includes continuous electrocardiogram and heart rate and intermittent blood pressure every 30 minutes for the first 2 hours of infusion (followed by monitoring by the institutional standard of care thereafter), continuous pulseoxymetry, and observation for any neurological symptoms suggestive of ketamine toxicity; this intervention is limited to the inpatient setting.
e. Lidocaine
Lidocaine is cited in pediatric oncology NP in two forms, IV lidocaine and 5% lidocaine patches [44, 49, 54]. IV lidocaine infusions rates of 9.3–14 mcg/kg/min (0.84 mg/kg/hr) were used in a case report in conjunction with IV ketamine for pain at the end of life [44]. Continuous intravenous lidocaine infusions are used in our pediatric oncology pain practice for refractory chronic NP and for acute episodes of NP related to antiGD2 antibody infusions for neuroblastoma therapy, in doses of 1–2 mg/kg/hr, for 4–8 hours. This option is usually considered after therapeutic trials with gabapentinoids and tricyclic antidepressants have not achieved full analgesic efficacy. Required monitoring includes continuous electrocardiogram and heart rate and intermittent blood pressure every 30 minutes, and observation for any neurological symptoms suggestive of lidocaine toxicity; this intervention may be used in the inpatient or outpatient setting (pain clinic).
In a 3 month prospective clinical trial of 12 patients and a case review of 5 patients, 5% lidocaine patches were shown to be efficacious [49, 54]. Pediatric and adult data both indicate 5% lidocaine patches for NP as every 12 hours applications (Table 4).
6. Considerations for Duration of Treatment
NP is notoriously difficult to treat and persistent in time; therefore, one should consider with caution the evidence for pharmacological interventions in view of the duration of treatment applied in studies. NP in pediatric oncology in the post-surgical setting, either limb sparing or amputation, has been noted to have a comparable long duration, of mean (SD) of 7.2 (8.4) weeks and 4.9 (4.0) weeks respectively, and 6.5 weeks (SD 7.2) for the entire group [27]. Medications specific for NP were administered for a mean (SD) duration of 9.5 (8.1) weeks (median, 7, range 1.7–30). In comparison, the open label pregabalin pilot study reported a duration of treatment of 8 weeks [42]. Given the persistent nature of NP extending over a significant length of time, it may be beneficial that prospective studies run over a prolonged time. An extended study time may also have positive implications by allowing sufficient time for dose escalation for NP specific medications like gabapentinoids and TCAs, which require slow dose escalation until analgesic efficacy is reached.
7. NP in Pediatric Oncology – Evidence for Nonpharmacological Treatment
In addition to pharmacological therapies for NP, non-pharmacological interventions for pain should be applied whenever possible. The nonpharmacological strategies would be strongly indicated in any context of chronic pain and furthermore in the circumstances of NP, which may have implications for quality of life, and may be associated with comorbidities of anxiety and depression [56]. Among nonpharmacological interventions for NP in pediatric oncology, the use of mirror therapy has been explored for phantom limb pain post amputation, in a retrospective investigation (n = 21) [46]. Other interventions may include acupuncture [57, 58] and music therapy [59].
There is emerging evidence for the efficacy of scrambler therapy (ST) for chemotherapy related NP in adults [60, 61] and in the pediatric population [62, 63], based on the fact that ST can block painful stimuli by sending “non-painful” stimuli to the cutaneous nerves. This electro-analgesia device consists of a multiprocessor that can generate non-painful stimuli by application of surface electrodes on skin over the painful areas. A prospective study of 9 pediatric patients (average age, 14 years and 2 months) with chemotherapy-induced peripheral neuropathy, indicated that the mean (SD) Numeric Rating Scale decreased from 9.22±0.83 to 0.11±0.33 after ST. While all patients entered the study on NP-directed medications including opioids, gabapentin, amitriptyline, and duloxetine, all but 2 patients were off medications by the end of the trial [63].
8. Conclusion
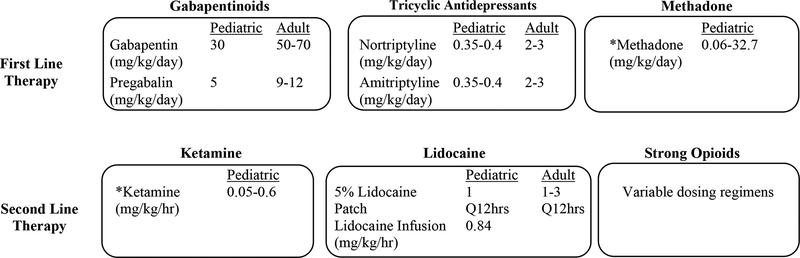
When comparing the pediatric doses to the adult doses of NP specific medications, the literature is suggestive of the possibility that pediatric populations may be under-dosed for treatment of NP. In the context of dose up titration for any medication used for NP, individual patient variables to be considered include the relative risk-benefit ratio, the individual response, and the clinical context. Consideration should be given to the possibility that dose escalation may be limited by side-effects. Medication classes used for the treatment of NP in adult and pediatric populations seem to be consistent, although SNRIs have not been evaluated in the pediatric population. Therefore, we recommend gabapentinoids and TCAs as first line therapy agents. Methadone, ketamine, and lidocaine may be useful adjuvants in selected patients. (Figure 1, medication dosing based on literature reflective of NP in pediatric oncology).
Figure 1.
NP in Pediatric Oncology - A Clinical Decision Algorithm
*Recommended for inpatient setting only
Although the literature suggests that analgesic efficacy can be reached in pediatric patients, given the small cumulative patient population, more research is necessary in pediatric NP. Most of the studies evaluated for pediatric oncology NP were case series or case reviews, with small sample sizes. Prospective studies extended over a substantial length of time are recommended based on the nature of NP as persistent, chronic pain and based on the need for sufficient time to escalate medication dose regimens to full analgesic efficacy. Combining non-pharmacological therapies with pharmacological interventions may be beneficial to the pediatric oncology population in achieving improved pain control and quality of life.
Supplementary Material
Key Points.
The treatment of neuropathic pain in pediatric oncology is challenging and often requires dose regimen escalation and addition of several lines of pharmacological interventions.
When comparing the algorithms for treatment of neuropathic pain in adults with those in children, the same concepts are applied.
Nevertheless, dose regimens in pediatrics seem to be modestly escalated in comparison with adult dose regimen, when adjusted for weight. Prospective multi-institutional studies in pediatric oncology may help elucidate the best combination regimens and generate specific dose escalation recommendations.
Acknowledgments
The authors have no conflicts of interest to declare. The preparation of this manuscript has been supported in part by the National Cancer Institute Cancer Center Support Core Grant 5P25CA023944 and ALSAC.
Funding for the work done by JMT was provided by the grant R25CA23944 from the National Cancer Institute.
Footnotes
Conflict of interest: DLA and JMT do not have any conflicts of interest to declare.
References
- 1.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008. April 29;70(18):1630–5. [DOI] [PubMed] [Google Scholar]
- 2.Hansson PT, Attal N, Baron R, Cruccu G. Toward a definition of pharmacoresistant neuropathic pain. Eur J Pain. 2009. May;13(5):439–40. [DOI] [PubMed] [Google Scholar]
- 3.Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat Med. 2011. July;25(5):553–9. [DOI] [PubMed] [Google Scholar]
- 4.Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, Maltoni M, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004. July 15;22(14):2909–17. [DOI] [PubMed] [Google Scholar]
- 5.Chandler A, Williams JE. Gabapentin, an adjuvant treatment for neuropathic pain in a cancer hospital. J Pain Symptom Manage. 2000. August;20(2):82–6. [DOI] [PubMed] [Google Scholar]
- 6.Keskinbora K, Pekel AF, Aydinli I. Gabapentin and an opioid combination versus opioid alone for the management of neuropathic cancer pain: a randomized open trial. J Pain Symptom Manage. 2007. August;34(2):183–9. [DOI] [PubMed] [Google Scholar]
- 7.Magnowska M, Izycka N, Kapola-Czyz J, Romala A, Lorek J, Spaczynski M, et al. Effectiveness of gabapentin pharmacotherapy in chemotherapy-induced peripheral neuropathy. Ginekol Pol. 2018;89(4):201–5. [DOI] [PubMed] [Google Scholar]
- 8.Mishra S, Bhatnagar S, Goyal GN, Rana SPS, Upadhya SP. A Comparative Efficacy of Amitriptyline, Gabapentin, and Pregabalin in Neuropathic Cancer Pain: A Prospective Randomized Double-Blind Placebo-Controlled Study. American Journal of Hospice and Palliative Medicine®. 2011. 2012/05/01;29(3):177–82. [DOI] [PubMed] [Google Scholar]
- 9.Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer. 2007. November 1;110(9):2110–8. [DOI] [PubMed] [Google Scholar]
- 10.Ross JR, Goller K, Hardy J, Riley J, Broadley K, A’Hern R, et al. Gabapentin is effective in the treatment of cancer-related neuropathic pain: a prospective, open-label study. J Palliat Med. 2005. December;8(6):1118–26. [DOI] [PubMed] [Google Scholar]
- 11.Fallon MT. Neuropathic pain in cancer. Br J Anaesth. 2013. July; 111(1):105–11. [DOI] [PubMed] [Google Scholar]
- 12.Jongen JL, Huijsman ML, Jessurun J, Ogenio K, Schipper D, Verkouteren DR, et al. The evidence for pharmacologic treatment of neuropathic cancer pain: beneficial and adverse effects. J Pain Symptom Manage. 2013. October;46(4):581–90. e1. [DOI] [PubMed] [Google Scholar]
- 13.Mishra S, Bhatnagar S, Gupta D, Nirwani Goyal G, Jain R, Chauhan H. Management of neuropathic cancer pain following WHO analgesic ladder: a prospective study. Am J Hosp Palliat Care. 2008 Dec-2009 Jan;25(6):447–51. [DOI] [PubMed] [Google Scholar]
- 14.Smith EM, Bridges CM, Kanzawa G, Knoerl R, Kelly JPt, Berezovsky A, et al. Cancer treatment-related neuropathic pain syndromes--epidemiology and treatment: an update. Curr Pain Headache Rep. 2014. November;18(11):459. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010. March;85(3 Suppl):S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard RF, Wiener S, Walker SM. Neuropathic pain in children. Arch Dis Child. 2014. January;99(1):84–9. [DOI] [PubMed] [Google Scholar]
- 17.Jacob E. Neuropathic pain in children with cancer. J Pediatr Oncol Nurs. 2004. Nov-Dec;21(6):350–7. [DOI] [PubMed] [Google Scholar]
- 18.Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol. 2014. July;29(7):932–7. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie Smith EM, Li L, Hutchinson RJ, Ho R, Burnette WB, Wells E, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013. Sep-Oct;36(5):E49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavoie Smith EM, Li L, Chiang C, Thomas K, Hutchinson RJ, Wells EM, et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst. 2015. March;20(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madden K, Bruera E. Very-Low-Dose Methadone To Treat Refractory Neuropathic Pain in Children with Cancer. J Palliat Med. 2017. November;20(11):1280–3. [DOI] [PubMed] [Google Scholar]
- 22.Walco GA, Dworkin RH, Krane EJ, LeBel AA, Treede RD. Neuropathic pain in children: Special considerations. Mayo Clin Proc. 2010. March;85(3 Suppl):S33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anghelescu DL, Faughnan LG, Jeha S, Relling MV, Hinds PS, Sandlund JT, et al. Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011. December 15;57(7):1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butkovic D, Toljan S, Mihovilovic-Novak B. Experience with gabapentin for neuropathic pain in adolescents: report of five cases. Paediatr Anaesth. 2006. March;16(3):325–9. [DOI] [PubMed] [Google Scholar]
- 25.Keskinbora K, Pekel AF, Aydinli I. The use of gabapentin in a 12-year-old boy with cancer pain. Acta Anaesthesiol Scand. 2004. May;48(5):663–4. [DOI] [PubMed] [Google Scholar]
- 26.Simon T, Niemann CA, Hero B, Henze G, Suttorp M, Schilling FH, et al. Short- and long-term outcome of patients with symptoms of spinal cord compression by neuroblastoma. Dev Med Child Neurol. 2012. April;54(4):347–52. [DOI] [PubMed] [Google Scholar]
- 27.Anghelescu DL, Steen BD, Wu H, Wu J, Daw NC, Rao BN, et al. Prospective study of neuropathic pain after definitive surgery for extremity osteosarcoma in a pediatric population. Pediatr Blood Cancer. 2017. March;64(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgoyne LL, Billups CA, Jiron JL Jr., Kaddoum RN, Wright BB, Bikhazi GB, et al. Phantom limb pain in young cancer-related amputees: recent experience at St Jude children’s research hospital. Clin J Pain. 2012. Mar-Apr;28(3):222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snaman JM, Baker JN, Ehrentraut JH, Anghelescu DL. Pediatric Oncology: Managing Pain at the End of Life. Paediatr Drugs. 2016. June;18(3):161–80. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Bhaskar A. Chemotherapy-induced peripheral neuropathic pain. BJA Education. 2016. 2016/04/01/;16(4):115–9. [Google Scholar]
- 31.Markman M. Chemotherapy-induced peripheral neuropathy: underreported and underappreciated. Curr Pain Headache Rep. 2006. August;10(4):275–8. [DOI] [PubMed] [Google Scholar]
- 32.Krumova EK, Geber C, Westermann A, Maier C. Neuropathic pain: is quantitative sensory testing helpful? Curr Diab Rep. 2012. August;12(4):393–402. [DOI] [PubMed] [Google Scholar]
- 33.Gilchrist LS, Tanner L. The pediatric-modified total neuropathy score: a reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer. 2013. March;21(3):847–56. [DOI] [PubMed] [Google Scholar]
- 34.Morgan KJ, Anghelescu DL. A Review of Adult and Pediatric Neuropathic Pain Assessment Tools. Clin J Pain. 2017. September;33(9):844–52. [DOI] [PubMed] [Google Scholar]
- 35.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007. December 5;132(3):237–51. [DOI] [PubMed] [Google Scholar]
- 36.Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006. November;13(11):1153–69. [DOI] [PubMed] [Google Scholar]
- 37.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007. Spring;12(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005. December 5;118(3):289–305. [DOI] [PubMed] [Google Scholar]
- 39.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015. February;14(2):162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finnerup NB, Attal N. Pharmacotherapy of neuropathic pain: time to rewrite the rulebook? Pain Manag. 2016;6(1):1–3. [DOI] [PubMed] [Google Scholar]
- 41.Fornasari D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017. December;6(Suppl 1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vondracek P, Oslejskova H, Kepak T, Mazanek P, Sterba J, Rysava M, et al. Efficacy of pregabalin in neuropathic pain in paediatric oncological patients. Eur J Paediatr Neurol. 2009. July;13(4):332–6. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen VF, Lundberg V, Jespersen TW, Hasle H. Extreme doses of intravenous methadone for severe pain in two children with cancer. Pediatr Blood Cancer. 2015. June;62(6):1087–90. [DOI] [PubMed] [Google Scholar]
- 44.Kajiume T, Sera Y, Nakanuno R, Ogura T, Karakawa S, Kobayakawa M, et al. Continuous intravenous infusion of ketamine and lidocaine as adjuvant analgesics in a 5-year-old patient with neuropathic cancer pain. J Palliat Med. 2012. June;15(6):719–22. [DOI] [PubMed] [Google Scholar]
- 45.Klepstad P, Borchgrevink P, Hval B, Flaat S, Kaasa S. Long-term treatment with ketamine in a 12-year-old girl with severe neuropathic pain caused by a cervical spinal tumor. J Pediatr Hematol Oncol. 2001. December;23(9):616–9. [DOI] [PubMed] [Google Scholar]
- 46.Anghelescu DL, Kelly CN, Steen BD, Wu J, Wu H, DeFeo BM, et al. Mirror Therapy for Phantom Limb Pain at a Pediatric Oncology Institution. Rehabil Oncol. 2016. July;34(3):104–10. [PMC free article] [PubMed] [Google Scholar]
- 47.Akkurt HE, Gumus H, Goksu H, Odabasi OF, Yilmaz H. Gabapentin Treatment for Neuropathic Pain in a Child with Sciatic Nerve Injury. Case Rep Med. 2015;2015:873157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown S, Johnston B, Amaria K, Watkins J, Campbell F, Pehora C, et al. A randomized controlled trial of amitriptyline versus gabapentin for complex regional pain syndrome type I and neuropathic pain in children. Scand J Pain. 2016. October;13:156–63. [DOI] [PubMed] [Google Scholar]
- 49.Orellana Silva M, Yanez V, Hidalgo G, Valenzuela F, Saavedra R. 5% lidocaine medicated plaster use in children with neuropathic pain from burn sequelae. Pain Med. 2013. March;14(3):422–9. [DOI] [PubMed] [Google Scholar]
- 50.Taylor M, Jakacki R, May C, Howrie D, Maurer S. Ketamine PCA for treatment of end-of-life neuropathic pain in pediatrics. Am J Hosp Palliat Care. 2015. December;32(8):841–8. [DOI] [PubMed] [Google Scholar]
- 51.Yazde Puleio ML, Gomez KV, Majdalani A, Pigliapoco V, Santos Chocler G. Opioid treatment for mixed pain in pediatric patients assisted by the Palliative Care team. Five years of experience. Arch Argent Pediatr. 2018. February 1;116(1):62–4. [DOI] [PubMed] [Google Scholar]
- 52.DeMoss P, Ramsey LH, Karlson CW. Phantom Limb Pain in Pediatric Oncology. Front Neurol. 2018;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anghelescu DL, Faughnan LG, Baker JN, Yang J, Kane JR. Use of epidural and peripheral nerve blocks at the end of life in children and young adults with cancer: the collaboration between a pain service and a palliative care service. Paediatr Anaesth. 2010. December;20(12):1070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nayak S, Cunliffe M. Lidocaine 5% patch for localized chronic neuropathic pain in adolescents: report of five cases. Paediatr Anaesth. 2008. June;18(6):554–8. [DOI] [PubMed] [Google Scholar]
- 55.Anghelescu DL, Faughnan LG, Hankins GM, Ward DA, Oakes LL. Methadone use in children and young adults at a cancer center: a retrospective study. J Opioid Manag. 2011. Sep-Oct;7(5):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012. June;16(3):191–8. [DOI] [PubMed] [Google Scholar]
- 57.Zeltzer LK, Tsao JC, Stelling C, Powers M, Levy S, Waterhouse M. A phase I study on the feasibility and acceptability of an acupuncture/hypnosis intervention for chronic pediatric pain. J Pain Symptom Manage. 2002. October;24(4):437–46. [DOI] [PubMed] [Google Scholar]
- 58.Butkovic D, Tot OK. Laser acupuncture treatment of neuropathic pain in a boy with brain tumour. Complement Ther Med. 2017. December;35:53–6. [DOI] [PubMed] [Google Scholar]
- 59.Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci Biobehav Rev. 2011. October;35(9):1989–99. [DOI] [PubMed] [Google Scholar]
- 60.Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage. 2012. January;43(1):87–95. [DOI] [PubMed] [Google Scholar]
- 61.Coyne PJ, Wan W, Dodson P, Swainey C, Smith TJ. A trial of Scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother. 2013. December;27(4):359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park HS, Kim WJ, Kim HG, Yoo SH. Scrambler therapy for the treatment of neuropathic pain related to leukemia in a pediatric patient: A case report. Medicine (Baltimore). 2017. November;96(45):e8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomasello C, Pinto RM, Mennini C, Conicella E, Stoppa F, Raucci U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: A preliminary study. Pediatr Blood Cancer. 2018. April 6:e27064. [DOI] [PubMed] [Google Scholar]
- 64.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. The Journal of biological chemistry. 1996. March 8;271(10):5768–76. [DOI] [PubMed] [Google Scholar]
- 65.Faria J, Barbosa J, Moreira R, Queiros O, Carvalho F, Dinis-Oliveira RJ. Comparative pharmacology and toxicology of tramadol and tapentadol. European journal of pain (London, England). 2018. May;22(5):827–44. [DOI] [PubMed] [Google Scholar]
- 66.Demant DT, Lund K, Finnerup NB, Vollert J, Maier C, Segerdahl MS, et al. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. Pain. 2015. November;156(11):2234–44. [DOI] [PubMed] [Google Scholar]
- 67.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. British journal of anaesthesia. 2011. October;107(4):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Intiso D, Basciani M, Santamato A, Intiso M, Di Rienzo F. Botulinum Toxin Type A for the Treatment of Neuropathic Pain in Neuro-Rehabilitation. Toxins. 2015. June 30;7(7).2454–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pierce DM, Shipstone E. Pharmacology update. tapentadol for neuropathic pain. The American journal of hospice & palliative care. 2012. December;29(8).663–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.