Figure 3.
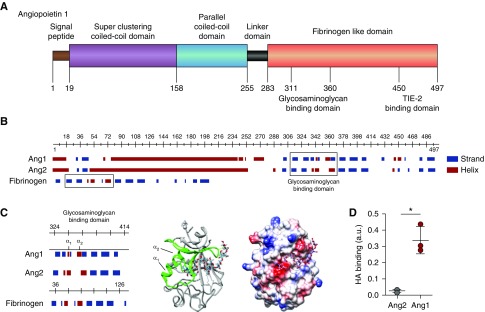
HA binds angiopoietin 1. (A) Structural characteristics of angiopoietin 1 (Ang1) reveal, besides the superclustering coiled-coil domain (purple) and parallel coiled-coil domain (blue) allowing Ang1 molecules to form clusters, a possible glycosaminoglycan- (GAG) binding domain within the N-terminal fibrinogen-like domain (orange) that neighbors the TIE2-binding site. (B) In contrast to the predicted GAG-binding domain (right box) of angiopoietin 2 (Ang2), the Ang1 GAG-binding domain resembles the HA-binding domain of fibrinogen (left box). (C) Like the predicted HA-binding Link module superfamily,17 the Ang1 GAG-binding domain contains two α-helices separated by an antiparallel β strand (left panel). The 3D ribbon configuration of the fibrinogen-like domain of Ang1 shows the position of the two α-helices in relation to the proposed HA-binding domain (middle panel), and the electrostatic surface projection of the 3D crystallographic structure reveals the positions of negative- (red), neutral- (white), and positive- (blue) charged sites within the HA-binding domain (right panel). (D) Direct binding of recombinant human Ang1 or Ang2 to HA. Values are given as mean±SD and difference was assessed by nonpaired two-tailed t test; *P<0.05.