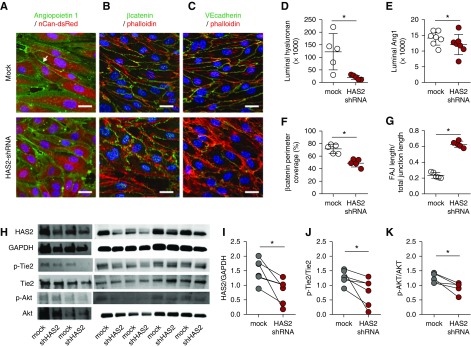
Figure 4.
HA angiopoietin 1 clustering stabilizes the endothelial layer. Representative cross-sectional confocal images of primary human glomerular-derived microvascular ECs (hgMVECs), transduced with an empty- (mock) or a HAS2-shRNA–containing lenti-viral construct (HAS2 shRNA) and exposed to a laminar shear stress of 5 dyne/cm2 for 3 days in the presence of 100 ng/ml recombinant human Ang1, stained (A) for HA (red) and Ang1 (green), revealed that especially at cell-cell contact areas (arrow) Ang1 presence is reduced, which is accompanied with (B) reduced membrane-bound active β-catenin (anti-ABC, green) and (C) increased unstable focal adherence junctions (FAJ) as visualized by VE-cadherin (CD144, green) staining. Both in combination with fluorescent-labeled phallotoxin (phalloidin-TRITC, red) to visualize intracellular F-actin and Hoechst 33258 (blue) nuclear stain (scale bar, 20 µm). Silencing of HAS2 results in reduced (D) HA expression, (E) Ang1 binding, (F) membrane-bound β-catenin, and (G) induction of unstable focal adherence junctions (FAJ) (n=5 per group). (H) Western-blot samples stained for HAS2, GAPDH, p-Tie2, Tie2, p-Akt, and Akt. Quantification of (I) HAS2 gene expression, (J) p-Tie2(Y992) over Tie2 expression, and (K) the downstream signaling Akt activation. Values are given as mean±SEM (D–G) or as individual data points (I–K). Differences between control and HAS2-shRNA–treated cells were assessed by paired two-tailed t test; *P<0.05.