Figure 4.
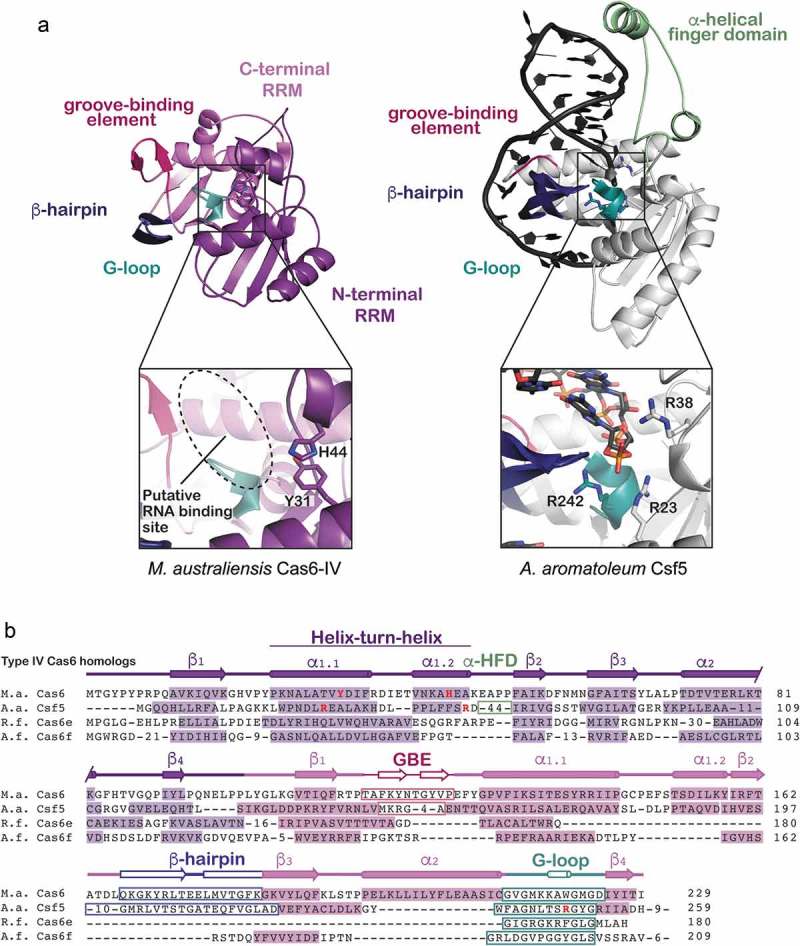
Structure and sequence alignments of Ma Cas6-IV with other Type IV RNA endonucleases. (a) A structural comparison of Ma Cas6-IV with the Cas6-homolog Csf5 from Aromatoleum aromaticum (PDB 6H9I). Features involved in binding crRNAs are indicated. The Csf5 protein contains a large insert called the alpha-helical finger domain (light green) that is not observed in Ma Cas6-IV. Residues predicted to activate cleavage of the crRNA are indicated in the inset below. (b) Sequence alignment of Ma Cas6-IV with other RNA endonucleases observed in Type IV systems. The N- and C-terminal RRM secondary structure elements are indicated, as well as features which bind crRNA, including the groove-binding element (GBE), beta-hairpin, and glycine-rich loop (G-loop). The alpha-helical finger domain (α-HFD) insert of Csf5 is also indicated. Active site residues of Ma Cas6-IV and Csf5 are bolded in red. Cas6e and Cas6f sequences are noticeably shorter than Ma Cas6-IV and Csf5, lacking large portions of the C-terminal RRM.
