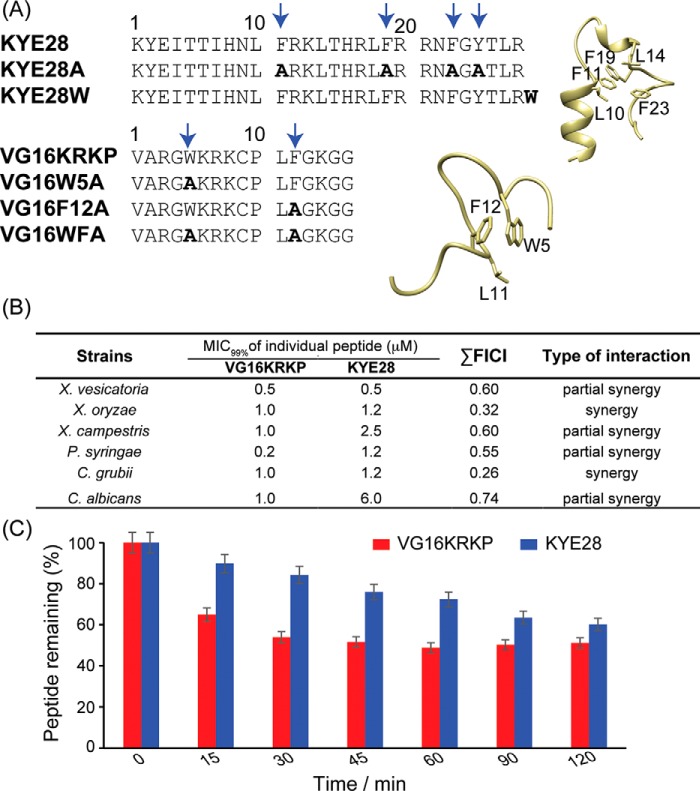
Figure 1.
A, primary amino acid sequences of the parent peptides, VG16KRKP and KYE28, as well as of their mutant analogs. The three-dimensional structure of the individual peptides in LPS is shown on right. The boldface amino acid residues are the mutated ones. B, checkerboard analysis using a modified micro broth dilution assay was performed to calculate the values of MIC99% and FICI. As shown, the parent VG16KRKP and KYE28 peptides interact with each other, demonstrated by the lowering of individual peptide concentration required for microbial killing. All experiments were performed in triplicate. C, proteolytic stability of the respective parent peptides, VG16KRKP and KYE28, in tomato plant extract containing plant proteases was evaluated using HPLC-based analysis. Both peptides displayed a half-life greater than 120 min. The peptides were incubated individually with the plant extract at 310 K, and aliquots were drawn at regular intervals and analyzed using RP-HPLC.