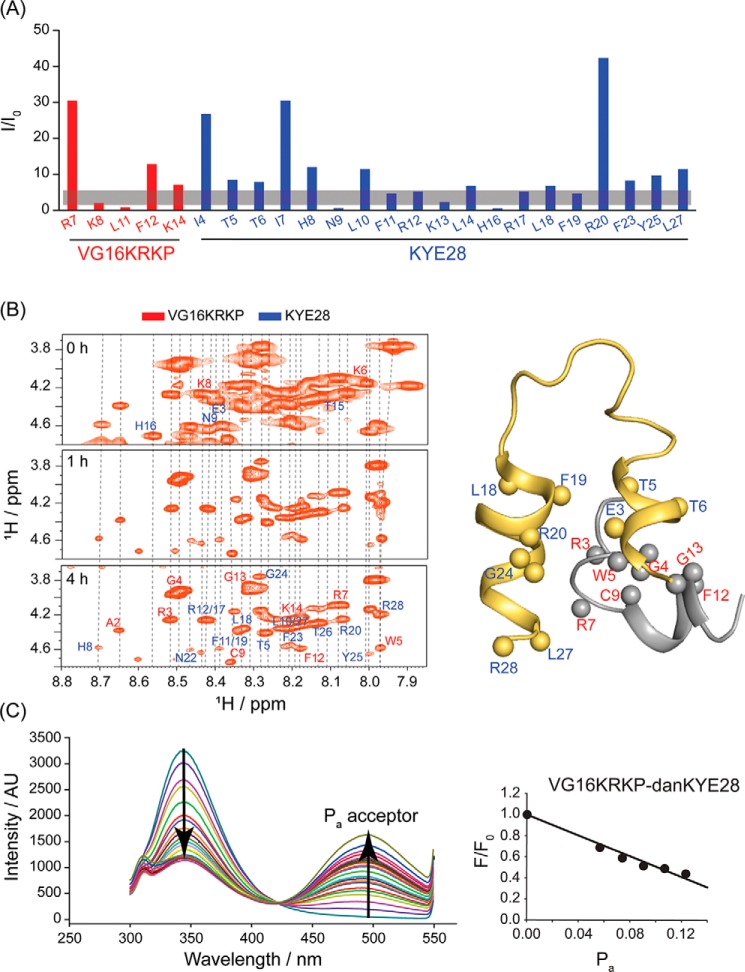
Figure 8.
A, PRE NMR was performed using MTSL-tagged VG16KRKP with KYE28 in LPS. The trNOESY experiment was conducted under similar conditions with the unlabeled peptide to compare residue-specific T2 relaxation, depicted as I/I0. The residues in the vicinity of the labeled Cys-9 residue exhibited almost complete relaxation as shown by broadened Hα/NH trNOESY peaks. B, similarly, H/D experiments performed throughout 5 h corroborated with the solved structure with residues involved in the formation of the hydrophobic hub and those engaged in π-cation interaction remaining shielded from the exchange kinetics. C, FRET experiment showed a linear change in the emission intensity of the Trp residue of the donor peptide, VG16KRKP upon titration with increasing concentrations of dansylated acceptor KYE28, upto 0.5 mol fraction. The FRET experiment was performed at 298 K in 10 mm potassium phosphate buffer (pH 7.4).