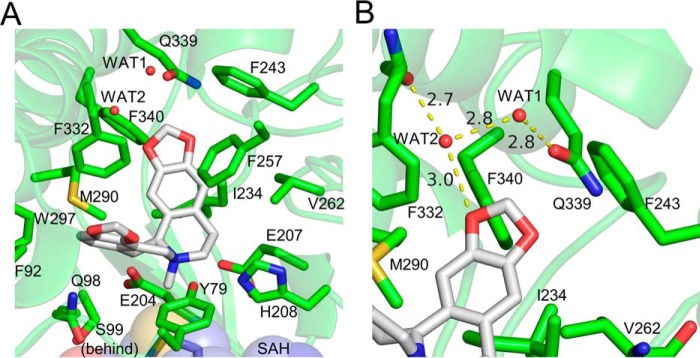
Figure 5.
Interactions between GfTNMT and the N-methylated product SMS. Residues within 4 Å of SMS are shown as lines and presented in all-atom representation. Hydrogen bonds are represented as yellow-dashed lines. A, SMS is held in a bent conformation within the binding-site pocket through hydrophobic interactions between the protein and the protoberberine backbone of SMS. B, presence of a hydrogen bond network between two water molecules and O-16 helps to recognize the terminal methylenedioxy bridge adjacent to ring A of the isoquinoline portion of the BIA substrate.