Figure 1.
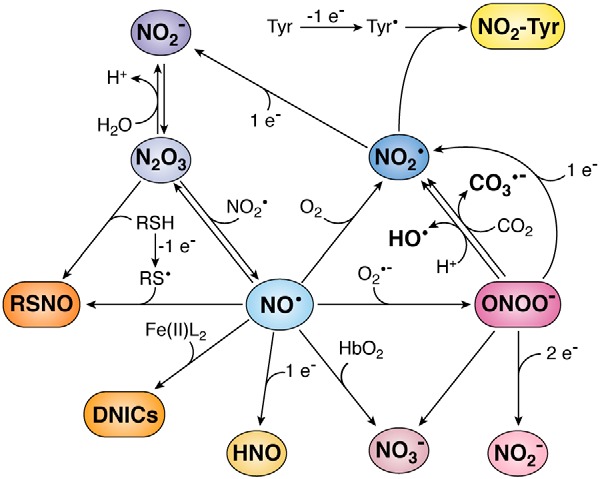
Nitric oxide and its biologically relevant derivatives. Nitric oxide can give rise to several species. Reaction with superoxide (O2•−) generates peroxynitrite (ONOO−); with oxyhemoglobin (HbO2), nitrate (NO3−); with oxygen (O2), nitrogen dioxide (NO2•); with strong one-electron reductants, nitroxyl (HNO); with liganded iron(II) (Fe(II)L2), dinitrosyl iron complexes (DNICs); with thiyl radical (RS•), S-nitrosothiol (RSNO); and with NO2•, dinitrogen trioxide (N2O3). Many of these products are reactive and yield further products. Peroxynitrite at neutral pH will protonate and generate NO3−, as well as NO2• and hydroxyl radicals (HO•) in 30% yield. In the presence of carbon dioxide (CO2), peroxynitrite will generate NO3−, as well as NO2• and carbonate anion radical (CO3•−) in 33% yield. In the presence of reductants, peroxynitrite will be reduced to nitrite (NO2−) or NO2•. Nitrogen dioxide can react with tyrosyl radicals (Tyr•) to generate 3-nitrotyrosine (NO2–Tyr) or with a reductant to form NO2−. Dinitrogen trioxide can be rapidly hydrolyzed to NO2−, it can be formed by NO2− in acidic pH, and it can react with thiols (RSH) to generate RSNO. In this figure, stoichiometries are not always strict, and protons are sometimes omitted for simplicity.
