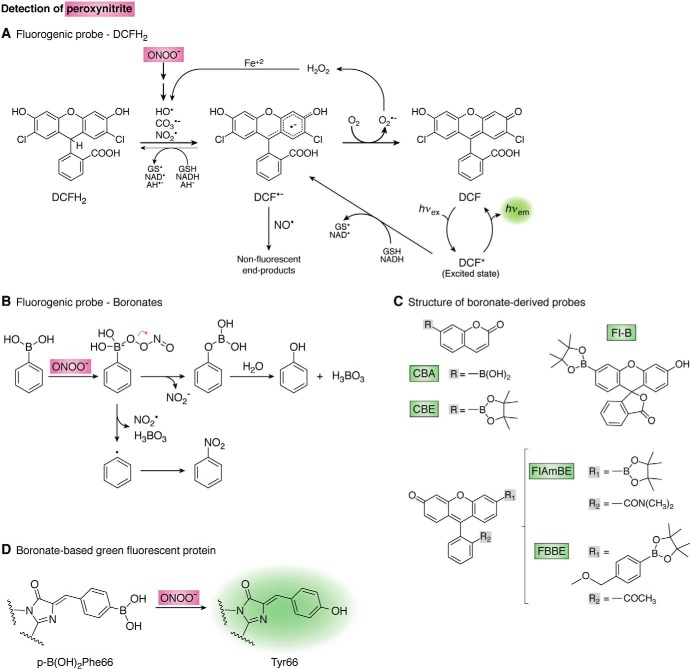
Figure 6.
Detection of peroxynitrite. A, mechanism of 2′,7′-dichlorodihydrofluorescein (DCFH2) oxidation. The reduced probe (DCFH2) is oxidized by peroxynitrite-derived radicals and other one-electron oxidants yielding a radical intermediate (DCF•−) that is oxidized by oxygen to yield fluorescent DCF. Thin arrows show alternative reactions and redox cycles. AH− stands for ascorbate. B, boronate oxidation by peroxynitrite. The major pathway (>85%, above) consists of heterolytic cleavage of the peroxyl bond leading to phenol which, appropriately derivatized, is fluorescent. The minor radical pathway (below) involves homolytic cleavage of the peroxyl bond giving NO2• and a phenyl-type radical that yields the nitro-derivative (201). C, structures of boronate-derived probes. CBA and CBE indicate a boronic acid or a boronic pinacolate ester attached to a coumarin scaffold, respectively (193). Fl-B (194), FlAmBE (192), and FBBE (205) are boronic pinacolate ester derivatives linked to a fluorescein scaffold with structural modifications as shown. D, genetically-encoded boronate-based GFP for the detection of peroxynitrite. Nucleophilic attack by the peroxynitrite anion to the phenylalanine boron moiety results in the formation of tyrosine and fluorescence. Modified from Ref. 176.