Fig. 2.
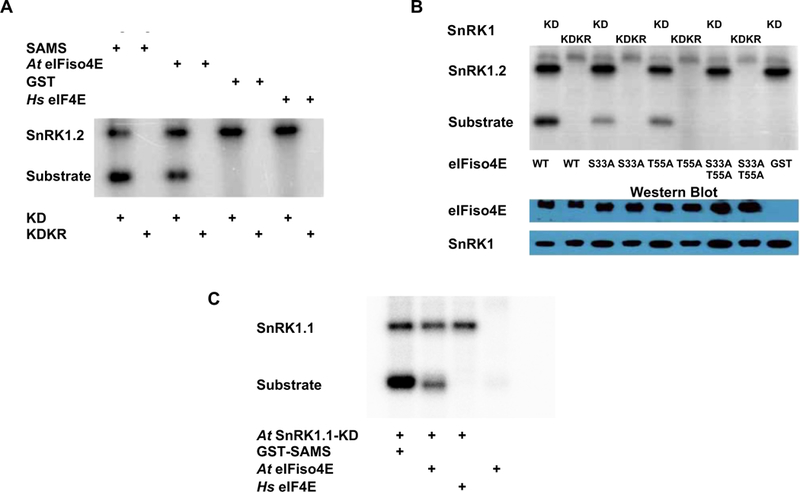
SnRK1 phosphorylates At eIFiso4E at serine 33 and threonine 55 in vitro. (A) Autoradiogram shows that the Arabidopsis SnRK1 kinase domain (KD, derived from SnRK1.2), but not inactive mutant SnRK-KDK49R (KDKR), is capable of autophosphorylation and phosphorylation of Arabidopsis eIFiso4E (At eIFiso4E) and positive control GST-SAMS, but not negative control proteins GST and human eIF4E (Hs eIF4E). HA2His6-tagged SnRK1-KD and SnRK1-KDKR were expressed in N. benthamiana, and the His6Xpress-tagged substrates At eIFiso4E and Hs eIF4E were expressed in E. coli. The indicated proteins were incubated together with γ32P-ATP, fractionated on SDS-containing polyacrylamide gels (SDS-PAGE), and exposed to a phosphor-imager to detect labeled proteins. (B) Autoradiogram shows that SnRK1-KD can phosphorylate wild type (WT) At eIFiso4E and single substitution mutants S33A and T55A, but not the double mutant S33A/T55A. Inactive SnRK1-KDKR and GST were included as negative controls. For loading controls, Western immunoblots were probed with anti-HA to detect the kinases, and anti-Xpress to detect At eIFiso4E and mutant derivatives. The autoradiogram shown is representative of three independent experiments, one of which included only the wild type and double mutant proteins. (C) Autoradiogram shows that SnRK1-KD derived from Arabidopsis SnRK1.1 is capable of autophosphorylation and also phosphorylates eIFiso4E and GST-SAMS, but not human eIF4E (Hs eIF4E).
