Fig. 4.
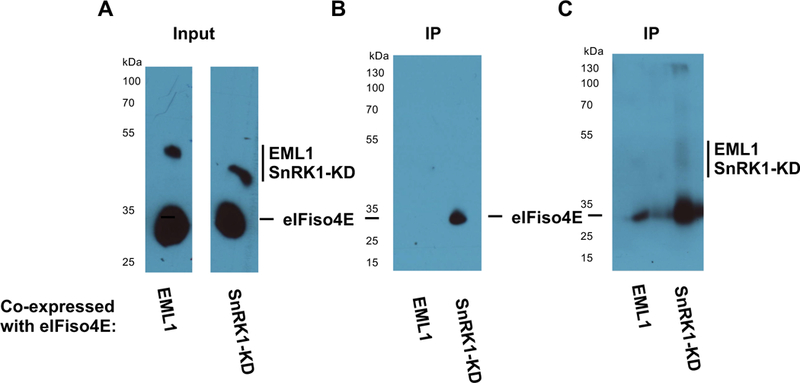
SnRK1 phosphorylates eIFiso4E in vivo.
(A) N. benthamiana leaves were infiltrated with agrobacterium cultures to co-express HA-His tagged eIFiso4E with similarly tagged SnRK1-KD or negative control protein EML1. Protein expression was verified by Western blot with HA antibody. (B) Phosphoproteins were immunoprecipitated from extracts using a phospho-antibody, and eIFiso4E was detected in immunoprecipitates with HA antibody. eIFiso4E was observed to immunoprecipitate with phospho-antibody when co-expressed with SnRK1-KD, but not with negative control protein EML1 when detection was performed using a picogram-level detection system. (C) The same blot shown in (B) was imaged using a femtogram-level detection system. Under these conditions, low-level phosphorylation of eIFiso4E was observed on co-expression with EML1, likely the result of endogenous kinase activity. Samples were loaded in every other lane, and signal in lanes flanking the eIFiso4E-SnRK1-KD is due to spill-over. The experiment shown is representative of three independent trials.
