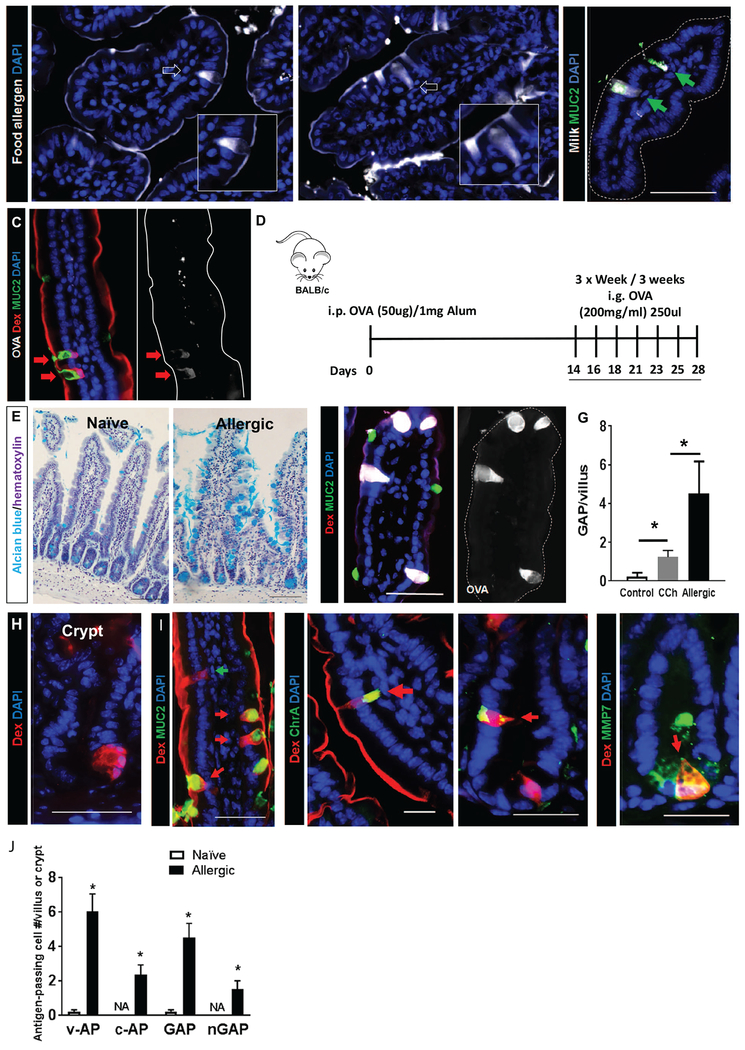
Figure 1. Antigen passage formation was dramatically increased and comprises multiple, secretory cell types in allergic mice.
(A) Immunofluorescence analysis of mouse small intestinal villus cross-sections that were exposed to milk or egg conjugated to Alexa Fluor 647 and stimulated with carbachol (CCh). White arrows point to antigen-positive intestinal epithelial cells that are morphologically identified as goblet cells shown in the insets. (B) Immunofluorescence analysis for MUC2 of mouse small intestinal villus cross-sections that were exposed to milk conjugated to Alexa Fluor 647 and stimulated with CCh. Green arrows point to MUC2+ GAPs with translocating Milk antigens. (C&F) Immunofluorescence analysis of mouse SIs from naïve, CCh-stimulated (C) and food allergic mice that are exposed to the food antigen OVA conjugated to Alexa Fluor 647 (F, Allergic-OVA). Both mice are also exposed to the imaging antigen dextran conjugated to rhodamine (Rh-Dex; “Dex” in figure) along with the food allergen. The dotted line indicates the apical edge of the SI epithelium. (D) Diagram depicting experimental design for developing food allergy. i.p.-intraperitoneal and i.g. intragastric. (E) Alcian blue stain with hematoxylin counterstain on naïve and food allergic mouse SIs to visualize mucin-producing goblet cells. (G) GAP quantitation of naïve (saline treated control and CCh treated) and food allergic allergen challenged mouse SIs; n = 3-4 per group. Student t-test, * indicates statistical significance. (H) Immunofluorescence cross-section of SI crypt from food allergic mice. Nucleus was visualized with DAPI (blue). (I) Immunofluorescence analysis for MUC2, chromogranin A (ChrA), enteroendocrine cell marker and metalloproteinase 7 (MMP7), Paneth cell marker in allergic mice that were challenged with OVA then exposed to Rh-Dex (Dex). (J) Quantitation of antigen-positive cells, including v-AP (villus antigen-passages), c-AP (crypt antigen-passages), GAP (villous MUC2+), and nGAP (villous MUC2−) cells in naive, saline-treated and food allergic allergen challenged mouse SIs; n = 3-4 per group. NA denotes zero value.