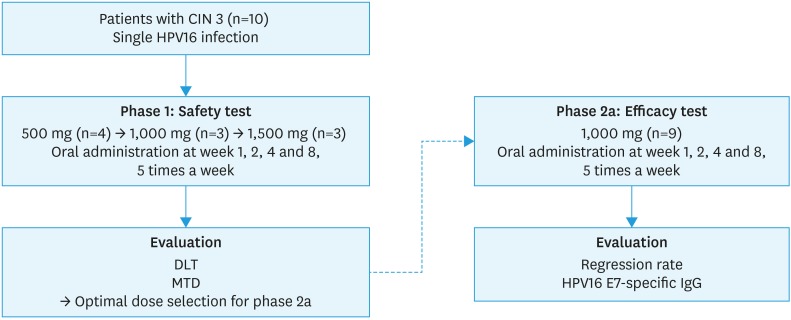
Fig. 1. Scheme of the phase I/IIa clinical trial. In the phase I trial, DLT and MTD were assessed after the first dose of treatment and each subsequent cohort was enrolled once each subject had completed the first dose of treatment. In the phase II trial, efficacy was assessed based on CIN by colposcopic biopsy.
CIN, cervical intraepithelial neoplasia; DLT, dose-limiting toxicity; HPV, human papillomavirus; IgG, immunoglobulin G; MTD, maximum tolerated dose.