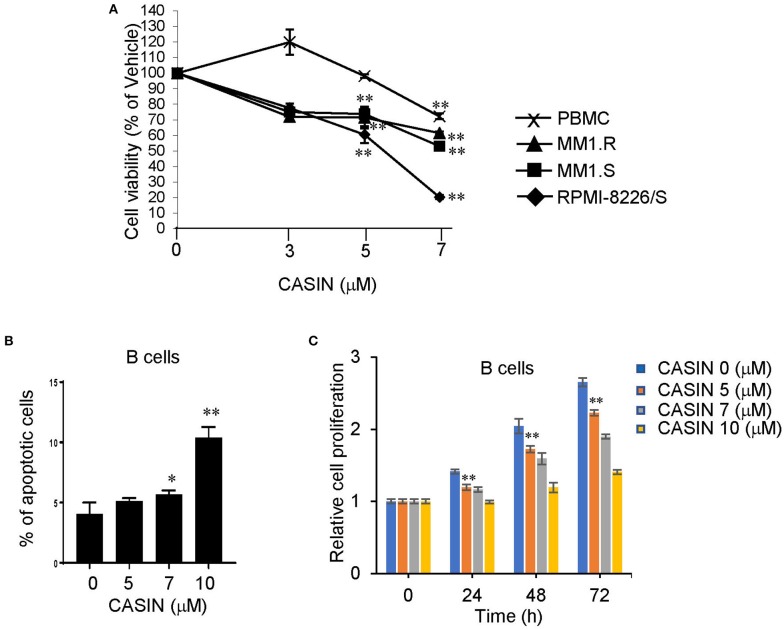
Figure 6.
CASIN has negligible side effects. (A) CASIN (5 μM) inhibits viability of MM cell lines but not healthy human peripheral blood mononuclear cells (PBMC). RPMI-8226/S, MM1.S, and MM1.R MM cells and PBMC were treated with or without the indicated concentrations of CASIN for 4 days. Viable cells were measured using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Data were normalized to Vehicle group (0 μM CASIN), **P < 0.01 vs. Vehicle group. (B) CASIN (5 μM) does not cause apoptosis of human B cells. Human B cells were treated with or without the indicated concentrations of CASIN for 2 days. Cell apoptosis was determined using flow cytometry analysis of Annexin V+ cells; *P < 0.05 and **P < 0.01 vs. Vehicle group. (C) CASIN (5 μM) has modest inhibitory effect on proliferation of human B cells. Human B cells were treated with or without the indicated concentrations of CASIN for the indicated time. Cell proliferation was then measured. **P < 0.01 vs. Vehicle group at same time point. Error bars represent means ± SD of triplicates and data are representative of three independent experiments.