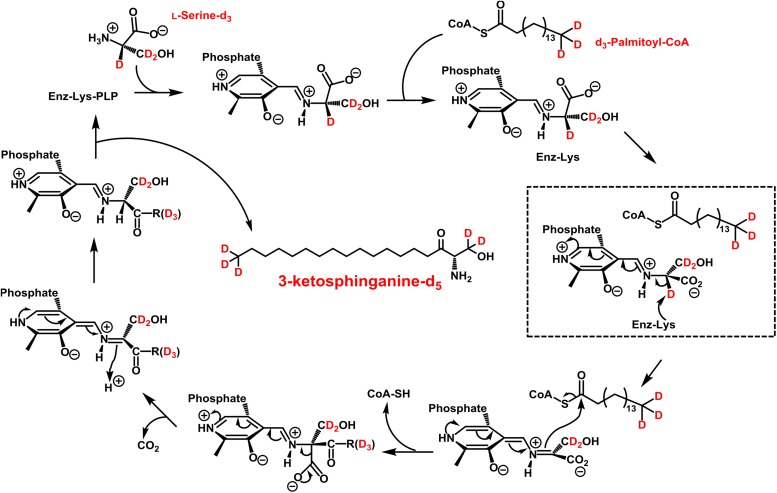
FIGURE 3.
Proposed catalytic cycle for serine palmitoyltransferase (SPT) with d3-palmitoyl-CoA and L-serine-d3 being the substrates. This scheme was modified from A. H. Merrill, Jr. (Merrill, 2011). An internal aldimine (Schiff base) formed between the cofactor pyridoxal 5’-phosphate (PLP) and an active site lysine residue (Enz-Lys-PLP, upper left) is replaced by the external aldimine through the incoming L-serine-d3. Binding of d3-palmitoyl-CoA results in transfer of serine α-deuterium to an enzymatic lysine residue (dashed box). The reaction proceeds as shown with free CoA, CO2, and 3-ketosphinganine-d5 (3KS-d5) are released as products.