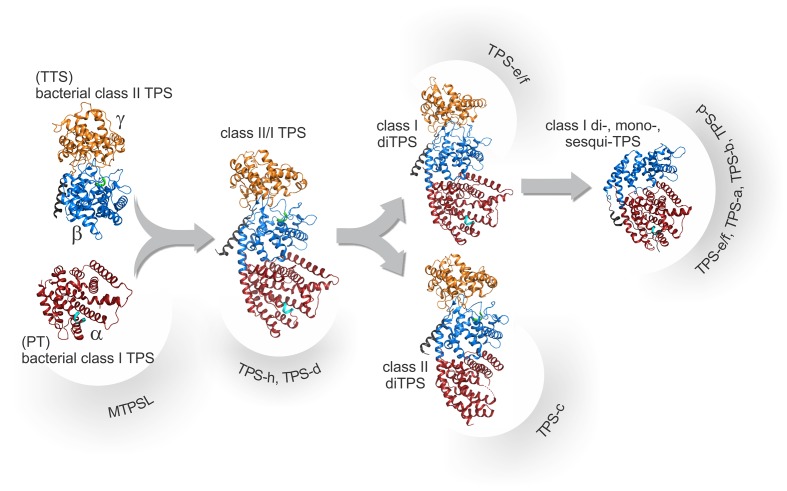
Figure 3.
Schematic overview of the proposed structural and evolutionary relationships among terpene synthases (TPSs) as based on known protein structures. Proposed progenitors of TPSs include ancestral bacterial class II diTPSs with a signature α-barrel βγ-domain harboring a catalytic DxDD motif, here exemplified by the ent-copalyl diphosphate synthase from Streptomyces platensis (PDB 5BP8; Rudolf et al., 2016). These, in turn, are related to ancestral triterpene synthases (TTS) and bacterial class I diterpene synthases (diTPSs) that adopt the signature α-domain structure with a conserved DDx2D motif, here exemplified by ent-kaurene synthase from Bradyrhizobium japonicum (PDB 4W4R; Liu et al., 2014) closely related to ancestral prenyltransferases (PT). Fusion of the ancestral monofunctional genes will have given rise to diTPSs with three helical domains (αβγ) represented here by Abies grandis abietadiene synthase (PDB 3S9V; Zhou et al., 2012a). Duplication and subsequent loss of activity in the βγ- and α-domains, respectively, lead to the emergence of monofunctional plant class II, here represented by Arabidopsis thaliana ent-copalyl diphosphate synthase, (PDB 4LIX; Köksal et al., 2014) and class I diTPSs, here represented by Taxus brevifolia taxadiene synthase (PDB 3P5P; Köksal et al., 2011). Through further loss of the γ-domain and various neo-functionalization and specialization events, the large classes of βα-domain class mono- and sesqui-TPSs will have arisen. Domain colors illustrate the γ-domain (orange), the β-domain (blue), the α-domain (red), as well as the conserved DxDD (green) and DDx2D (cyan) motifs.