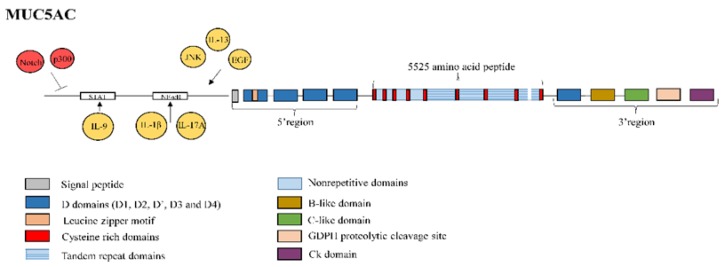
Figure 4.
Structure of mucin MUC5AC. MUC5AC is a polymeric mucin made of 5,525 amino acids. The 5′-flanking region consists of the cysteine rich domains D1, D2, D’ and D3 and a putative leucine zipper motif. The central region is encoded by a single large exon containing 9 cysteine rich domains of which cysteine 1 to cysteine 5 domains are interspersed by nonrepetitive sequences and cysteine 5 to Cysteine 9 domains are interspersed by four tandem repeat domains. Only the last repetitive domain shows a slight variation in length. The 3′-flanking region consists of the cysteine rich domains D4, B, C and cysteine knot (CK). Moreover, a GDPH (Gly-Asp-Pro-His) autocatalytic proteolytic cleavage site is also present in this region. MUC5AC promoter activity can be downregulated by the histone acetyltransferase p300 and the Notch signalling pathway. MUC5AC promoter activity can be upregulated by autophagy, which is defective in IPF, through phosphorylation of Jun N-terminal kinase (JNK) and c-Jun, by interleukin (IL)-13, and by epidermal growth factor (EGF). IL-1β and IL-17A, through nuclear factor kappa B (NF-kB) binding site in the MUC5AC promoter, and IL-9, via the Janus kinase/signal transducers and activators of transcription (JAK/STAT) patway, also upregulate MUC5AC.