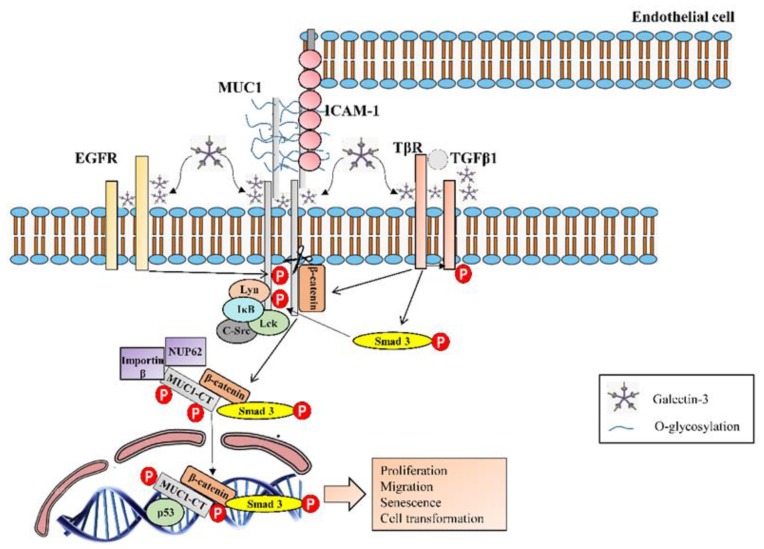
Figure 8.
MUC1 activation and intracellular signalling. MUC1 is activated by extracellular ligands such as the intracellular adhesion molecule-1 (ICAM-1), which interacts with the protein component of the MUC1 VNTR or MUC1-N terminal domain. MUC1-C interacts with the galectin 3 ligand, which functions as a bridge to associate MUC1-C with different growth factor receptors at the cell surface as for example epidermal growth factor receptor (EGFR), which can activate and phosphorylate MUC1-CT. For the transforming growth factor β1 (TGFβ1) receptor (TβR), galectin-3 stabilizates it, so galectin-3 could serve also as a bridge between MUC1-C and TβR. Moreover, it has been hypothesized that through Smad3 phosphorylation, induced by TβR activation, MUC1-CT is phosphorylated and translocated into the nucleus. Furthermore, TGFβ1 is also able to activate and induce nuclear localization of the fibrotic transcription factor β-catenin. A nuclear protein complex constituted by MUC1-CT/phospho (p)-Smad3 and β–catenin is formed and it promotes fibrotic processes such as epithelial to mesenchymal transition (EMT) and fibroblast to mesenchymal transition (FMT). MUC1-CT can also directly interact with intracellular kinases such as c-Src, Lyn and Lck or IκB kinase complex, which also activate and phosphorylate MUC1-CT. Phosphorylated MUC1-CT interacts with importin-β and with nucleoporin p62 (Nup62) to migrate into the nucleus. Once in the nucleus, MUC1-CT forms also nuclear complexes with other proteins as for example p53 and mediates IPF-linked processes such as proliferation, migration, cell senescence and cell transformation.