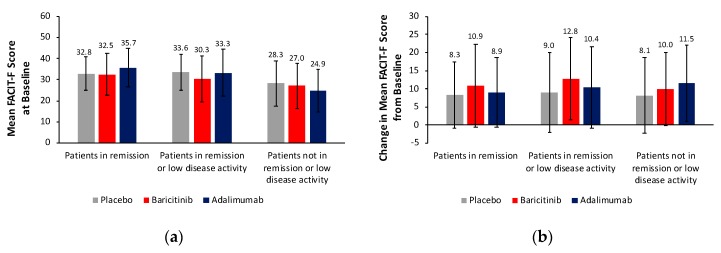
Figure 4.
FACIT-F scores in patients from RA-BEAM by remission status (a) at baseline and (b) change at week 24. Error bars indicate standard deviation. FACIT-F scores at baseline and change in FACIT-F scores based on numbers of patients from RA-BEAM in remission (DAS28-ESR <2.6): PBO+MTX n = 24, BARI+MTX n = 87, ADA+MTX n = 57; in remission or low disease activity (DAS28-ESR ≥2.6 and ≤3.2): PBO+MTX n = 46, BARI+MTX n = 154, ADA+MTX n = 110; and not in remission or low disease activity (DAS28-ESR >3.2): PBO+MTX n = 276, BARI+MTX n = 266, ADA+MTX n = 156. One patient was missing from the BARI+MTX group and one from the ADA+MTX group for patients not in remission or low disease activity. ADA adalimumab, BARI baricitinib, DAS28-ESR Disease Activity Score for 28-joint count with erythrocyte sedimentation rate, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, MTX methotrexate, PBO placebo.