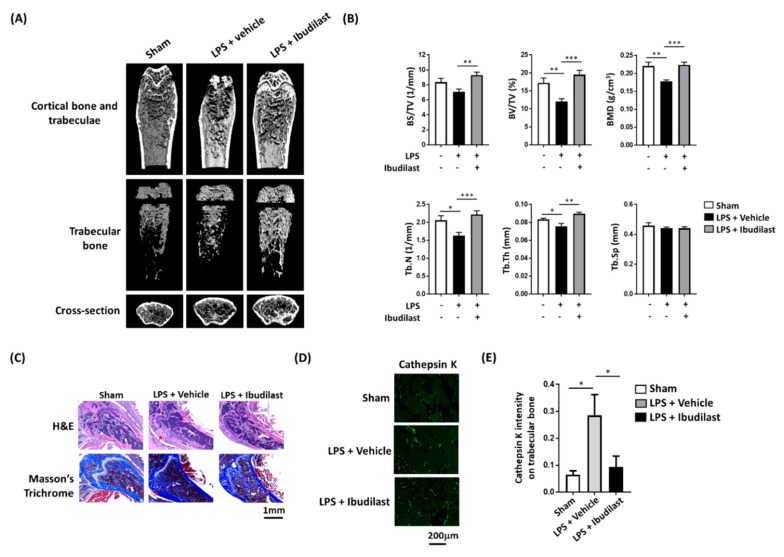
Figure 5.
Intraperitoneal administration of ibudilast following intrafemoral injection of LPS protects mice against LPS-induced alteration in bone morphology and prevents osteoclast activation. (A) At one hour after intrafemoral LPS injection, mice received intraperitoneal ibudilast or PBS (vehicle) once daily for 3 consecutive days. Micro-CT images revealed that ibudilast exerts protective effects against LPS-induced reduction of bone density. (B) Quantitative data analysis confirmed these findings. (C) Hematoxylin/eosin and Masson’s trichrome staining revealed a dense bone morphology in PBS-treated mice, whereas LPS injection resulted in bone loosening (black arrow)—a finding which was attenuated by ibudilast administration. (D) Immunofluorescence was used to detect cathepsin K (an osteoclast marker). LPS increased cathepsin K fluorescence intensity—a finding which was attenuated by ibudilast administration. (E) Quantitative data analysis confirmed these findings. Data are presented as means ± standard errors of the mean. Analyses were conducted with two-way ANOVA followed by Tukey’s post-hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001. LPS, lipopolysaccharide; PBS, phosphate-buffered saline.