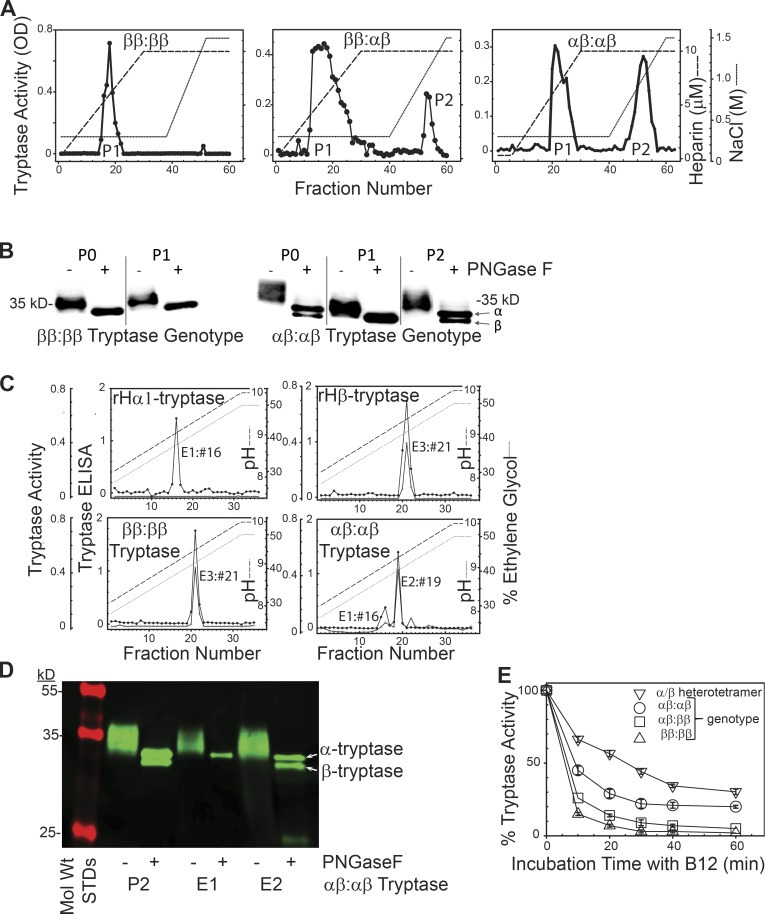
Figure 2.
Separation and stability of tissue-derived β-tryptase homotetramers and α/β-tryptase heterotetramers. (A) B2-agarose–purified tryptase (P0) from tryptase-genotyped donors and subjected to phosphocellulose chromatography. Representative of tryptase prepared from three individuals with each genotype. (B) Western blotting (G3 anti-tryptase mAb) after SDS-gradient PAGE of P0 and P1 and P2 fractions from A, after being ±N-deglycosylated (PNGase F), reduced (DTT), and heat-denatured (SDS). Representative of experiments from two individuals with each genotype. (C) G5-Sepharose immunoaffinity chromatography of mature rH-α1-tryptase (left upper panel), rH-β2-tryptase (right upper panel), and lung tryptase (ββ:ββ genotype, P0, left lower panel; αβ:αβ genotype, P2, right lower panel). Representative of two individuals with each genotype. (D) SDS-PAGE (10–20%) of P2, E1, and E2 fractions (αβ:αβ genotype) obtained as above from one of the two subjects with an αβ:αβ genotype. (E) Stability of α/β-tryptase heterotetramer (P2, ββ:αααβ genotype), β-tryptase (P0, ββ:ββ genotype), and mixtures of α/β-tryptase and β-tryptase (P0, αβ:ββ or αβ:αβ genotypes) to inhibition by B12 anti-tryptase mAb. Mean ± SD. Data reflect tryptase from three different individuals for each of the three genotypes displayed.