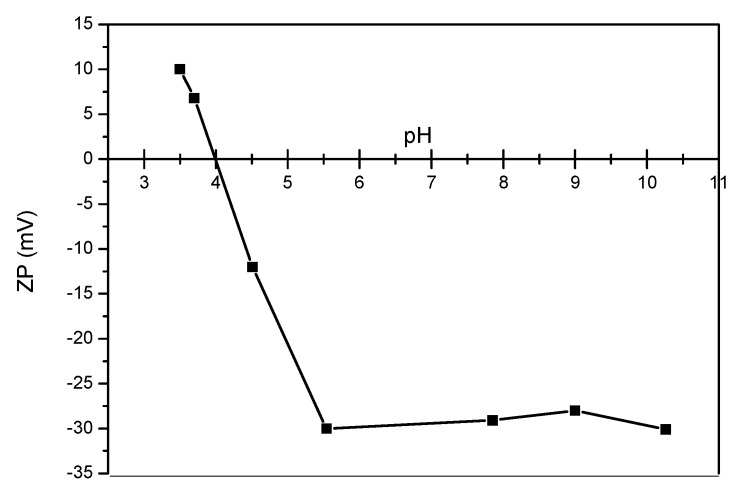
Figure 9.
Effect of the solution pH on ζ-potential of MNPs for sample A. Increasing the pH decreases the positive charge as some of the carboxyl groups become negatively charged. At pH 4.1, the potential decreases to zero. Further increasing the pH beyond the isoelectric point increases the negative charge until pH 5.5 where the zeta potential reaches plateau.