Abstract
Context: Urinary tract infections (UTI) are the most frequent secondary health condition following spinal cord injury or disease (SCI/D) that adversely impact overall health and quality of life, and often result in rehabilitation service interruptions, emergency department visits, and urinary sepsis.
Methods: Experts in Urohealth and/or UTI recognition and management and the SCI-High Project Team used a combination of evidence synthesis and consensus methods for developing the UTI indicators. A systematic search and a Driver diagram analysis were applied to identify key factors influencing UTI. This Driver diagram guided the UTI Working Group when defining the construct, specifying the aim for the UTI SCI/D quality indicators, and developing the UTI diagnostic checklist and fever definition.
Results: The structure indicator was the proportion of patients with a health care professional (i.e. family physician or urologist) able to follow-up with the patient regarding urine culture and sensitivity results within 48–72 h of collection. The Working Group knowingly adopted a single checklist for UTI diagnosis, recognizing the stark contrast in the complexity of diagnosis in acute versus community settings. The process indicator is the proportion of SCI/D rehabilitation inpatients with UTI as defined by the UTI diagnostic checklist. The outcome indicator is the proportion of SCI/D rehabilitation inpatients with inappropriate antibiotic prescription.
Conclusion: UTI can be diagnosed using the developed symptoms and signs checklist. These structure, process, and outcome quality indicators will ultimately reduce inappropriate antibiotic therapy for UTI and the rising incidence of antibiotic resistance among community-dwelling individuals with chronic SCI/D.
Keywords: Spinal cord injuries, Healthcare quality indicator, Rehabilitation, Urinary tract infection
Introduction
Spinal Cord Injury or disease (SCI/D) results in a complex constellation of motor, sensory and autonomic impairments. These include the development of Neurogenic Lower Urinary Tract Dysfunction (NLUTD),1 and the associated alterations in the ability to perceive bladder filling, and complete voluntary efficient voiding. The goals of urohealth management during rehabilitation are to: (1) achieve continence with routine socially acceptable bladder emptying; (2) avoid urinary stasis, high filling and voiding pressures, which can lead to renal damage; and, (3) reduce the frequency and severity of urinary tract infection (UTI), prevent stones in the bladder/kidneys, urethral trauma or stricture, and autonomic dysreflexia (due to bladder distension).2 Among these conditions related to NLUTD following SCI/D, UTIs are the most common.3 The current definition of UTI in persons with NLUTD requires the presence of leukocyturia, bacteriuria, and clinical symptoms.4,5 UTIs are a frequent cause of rehabilitation service interruptions due to change(s) in the individual's health status.6 Further, UTIs result in frequent appropriate and inappropriate emergency department visits.7 A UTI during the immediate post-injury acute care hospitalization increases the cost of rehabilitation admission by 5,388 CAD.8 A UTI associated with severe urosepsis (fever, hypotension, systemic inflammation, and organ dysfunction due to an infection originating from the urinary tract)9 can result in an immune-deficiency syndrome increasing the individual's future risk of recurrent UTI,10 and adversely impacting functional outcome at five years post-injury.11 Thus, recognition and management of UTI is a priority for health system payers, rehabilitation service providers and stakeholders alike, to enhance rehabilitation and health outcomes among individuals with SCI/D.
There is significant controversy in the field as to what constitutes a UTI. Although there is consensus that the term “UTI” refers to significant bacteriuria among individuals with SCI/D and NLUTD, with symptoms or signs of infection. For example, most clinicians agree fever is a symptom of UTI,12 although health care providers use a variety of less established symptoms and signs to diagnose UTI, many of which have low sensitivity and specificity for UTI diagnosis.13 Previously, thought leaders in the field have proposed that UTI is an umbrella term which represents a “heterogeneous group of clinical diagnoses” that encompasses several clinical entities including urethritis, vaginitis, interstitial cystitis, pyelonephritis, etc. Further, catheter-associated UTI rates vary by the infection definition and the method of bladder drainage.14 To further conflate the lack of clinical clarity regarding UTI diagnosis, many studies report different colony count criteria for defining bacteriuria, without distinguishing symptomatic from asymptomatic patients.15 In response to the terminology conundrums, the European Association of Urology (2017) has developed and disseminated several UTI definitions for uncomplicated UTIs, complicated UTIs, recurrent UTIs, catheter-associated UTIs, and urosepsis in their most recent Urological Infections Guidelines.9
Individuals with SCI/D often confuse non-specific symptoms (e.g. fatigue, increased limb spasticity, etc.) with a UTI and request or initiate antibiotic therapy prior to urinalysis or culture confirmation of UTI diagnosis. This has led to over-treatment of UTI with antibiotics in SCI/D patients, and the emergence of antibiotic resistance. Day-to-day recognition and management of UTI is further complicated by antimicrobial use and failure of antimicrobial therapy for confirmed UTI. Dow et al.16 have described microbiological relapse in patients with SCI and lower urinary tract dysfunction and symptomatic relapse after a 3-day course of antibiotics. Their findings indicate that treatment for 14 days leads to improved clinical and microbiological outcomes. The United States Veterans Administration has reported dramatic rises in Gram-negative bacterial (GNB) resistance over time. In a retrospective cohort study among 19,421 veterans with SCI/D, 157,446 urine cultures with 216,504 bacterial isolates were reviewed. There was a proportionate increase of GNB over time, suggesting a growing burden of colonization and infection in the SCI/D population. In addition, 40% of GNB were fluoroquinolone-resistant. In response, there have been calls for stringent antibiotic stewardship programs for individuals with SCI/D.17 These programs are intended to reduce improper use of antibiotics and prevent the adverse effects of inappropriate antibiotic use including toxicity, colonization with multi-resistant bacterial and Clostridium difficile infection.17,18
A majority of Canadian inpatient tertiary rehabilitation hospitals have some form of local antibiotic stewardship program; however, no single entity provides provincial or national oversight programs in the outpatient setting after rehabilitation discharge.
Early recognition and definitive management of UTI are crucial to advancing the quality of care for Canadians living with SCI/D. However, there is no national or provincial system to track the quality of UTI care due to a lack of (1) systematic documentation of UTI diagnosis and, (2) definition for UTI resolution. In order to realize advances in UTI care, changes in the health system are required at the health systems, organizational level, and patient care levels.
Implementation of indicators is one approach to tracking the quality of care from a variety of perspectives. Indicators are explicitly defined measurable elements of practice performance, for which there is evidence or consensus support.19 Indicators of quality care can be categorized as structure, process, or outcome indicators.20,21 This manuscript describes the development of a framework of structure, process and outcome indicators to advance the quality of SCI/D rehabilitation care in the Domain of Urinary Tract Infection, from the time of rehabilitation admission to 18 months thereafter. This process is a part of the SCI-High Project (www.sci-high.ca) which aims to advance SCI/D rehabilitation care by 2020 through the selection, implementation, and evaluation of prioritized quality indicators in Canada.
Methods
A detailed description of the overall SCI-High Project methods and process for identifying UTI as a priority Domain for SCI/D rehabilitation care are described in related manuscripts in this issue respectively.22,23 In addition to the Project Team, an External Advisory Committee, National Data Strategy Committee, and the local Quality Committee/spinal cord rehabilitation leadership team supported the global project goals and provided oversight regarding the context for selection and implementation of quality indicators.
The approach to developing the Urinary Tract Infection Domain structure, process and outcome indicators followed a modified but similar quality improvement approach to that described by Mainz,24 which included the following planning and development phases: (a) formation and organization of the national and local Working Groups;23 (b) defining the Domain construct and specific aim; (c) providing an overview/summary of existing evidence and practice; (d) developing and interpreting a Driver diagram; (e) selecting indicators; and, (f) pilot testing and refinement of the Domain-specific structure, process, and outcome indicators. Throughout these aforementioned processes, meeting minutes and action items were recorded, and facilitated groups discussions occurred to achieve consensus amongst the Domain-specific Working Group and the SCI-High Project Team to utilize the Working Group member's relevant expertise on the topic while ensuring the broader goals of the SCI-High Project were aligned across the other Working Groups.
Urinary Tract Infection Working Group
Experts in Urohealth and/or UTI recognition and management were invited to participate in the SCI-High Project as members of the Urinary Tract Infection Domain-specific Working Group based on their practical or empirical knowledge of SCI/D rehabilitation, UTI, neuro-urology, and health service delivery. The Working Group was composed of 3 urologists, 2 physiatrists, 1 clinical nurse specialist and 2 registered nurses, 2 postdoctoral fellows, 1 family physician and 1 stakeholder with lived experience and expertise in primary care data interpretation. The Working Group met nine times via conference call totaling more than 13 h of discussion to define the key Construct, Aim and develop the indicators, and for an additional two hours thereafter, to refine the indicators based on pilot data feedback and to discuss manuscript preparation. The calls were recorded, meeting minutes and action items, were circulated after one call and before the next call. The Working Group's efforts were informed by concurrent collaborative initiatives including (1) development of new Canadian Urology Guidelines;25 and (2) the Ontario Neurotrauma Foundation-funded Ontario SCI Research Network Urohealth Summit and related white paper.26 In addition, individual Working Group members completed their own independent review of the prepared materials, shared with one another resources and or practice standards via email or teleconference, and conducted independent pilot implementations and evaluations of the proposed indicators outside of the formally scheduled meetings.
Driver diagram, aim, and construct definition
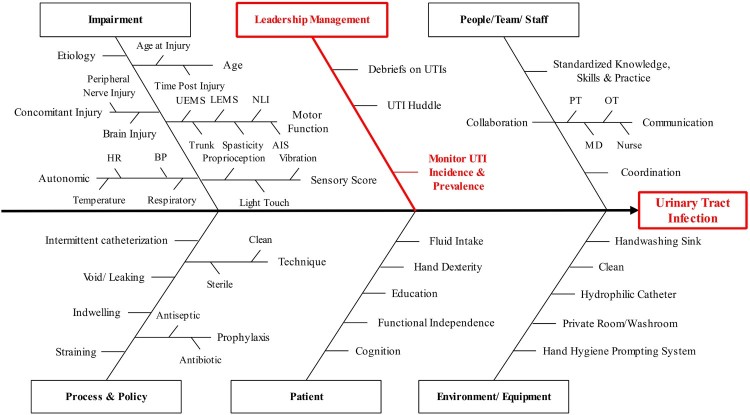
We used a combination of evidence synthesis and consensus methods for developing the UTI indicators. The process involved a systematic search to gather information about SCI rehabilitation care related to UTI and factors that influence UTI incidence, and a scoping synthesis of the data acquired. The Medline, and Embase databases were searched using the terms “Urinary Tract Infection” and “Spinal Cord Injury”. Non-English publications, UTI during the acute phase of SCI, non-causal effect studies, narrative reviews and conference abstracts were excluded. This information was then compiled in a Driver diagram (Figure 1). A Driver diagram is a visual display of a high-level quality improvement goal, and a set of underpinning factors/goals. The tool helped to organize change concepts as the Working Group discerned “what changes can we make, that will result in goal achievement in SCI/D rehabilitation care context”. The Driver diagram can also serve as a framework for monitoring progress toward goal attainment. The impairment branch of the Driver diagram was common across all SCI-High Project Domains. The branch of the Driver diagram noted in red represents the foci for development of the UTI indicators based on expert opinion. The Driver diagram guided the UTI Working Group when defining the central Construct and choosing the Aim for the development of the quality indicators and selecting Drivers for the outcome of interest.
Figure 1.
Driver diagram for the Urinary Tract Infection Domain. Boxes and letters in red represent the most feasible branches for indicator development according to the opinion of the Domain-specific Working Group. An individual's impairments, including their neurological level and completeness of injury, and degree of motor, sensory and autonomic dysfunction impact their NLUTD and the frequency and severity of UTI. UEMS: Upper-extremity motor score, LEMS: lower-extremity motor score, NLI: neurological level of injury, AIS: ASIA Impairment Scale; HR: heart rate, BP: blood pressure, PT: physiotherapist, OT: occupational therapist, MD: medical doctor.
Prior to coming to consensus, the UTI Working Group explored other drivers of importance including funding for catheters, the merits of single-use versus multiple-use catheters, and the role of hydrophilic catheters as drivers for reducing UTI incidence as goal. However, given there was substantial inter-provincial variation in catheter funding models and product availability, together with the concurrent conduct of an independent economic review of catheter funding by Health Quality Ontario,27 the Working Group deemed the catheter issues as secondary in importance to the central issue of UTI diagnosis. The Working Group anticipated that the economic rationale for catheter funding would become self-evident once there was good data describing UTI incidence and prevalence across the country.
Prior to proceeding with the development of indicators, the Working Group spent an inordinate amount of time (7 meetings) trying to define a UTI, the group discussions were consistent with the aforementioned conundrums in the field regarding UTI diagnosis. Although the group was able to rapidly define their aims (as shown below), they opted to spend the bulk of their time developing an operative definition of UTI. Development of the UTI definition entailed several distinct activities: (1) developing a definition of fever in the inpatient and outpatient settings based on review of available literature and current clinical practice (measurement of tympanic temperatures); (2) conducting a 3 month prospective quality practice project to define the sensitivity and specificity of signs and symptoms of UTI in an inpatient setting;13 (3) discussing and establishing a consensus acknowledgement of the differences in UTI presentation in the subacute hospital setting or chronic community setting; (4) conducting a prospective evaluation of the UTI definition in a rapid cycle quality improvement context in the outpatient setting at five centers across Canada (London, Toronto, Calgary, Edmonton and Winnipeg). As the definition of UTI was developed, the Working Group recognized the need for a decision tree acknowledging next steps in the event of UTI identification.
Selection of indicators
The Working Groups were asked to develop/select at least one structure, process and outcome indicator related to the Domain of UTI. Structure indicators are defined by the properties of the setting, in which the health care occurs.20 Process indicators describe what is actually done in giving and receiving care, while an outcome indicator reflects the patient's mortality, morbidity, health status, health-related quality of life or satisfaction with life within the context of the care provided.20 The Project Leaders stipulated that the indicators must be relevant, concise and feasible (10 min or less to implement), and aligned in their aim across the structure, process and outcome indicators to achieve a single substantive advance in SCI/D rehabilitation care. The Working Group was advised that they could use established measurement tools or developing their own (i.e. questionnaires, data collection sheets, laboratory exams, and medical record data), depending on the requirements and feasibility of a given indicator.
Results
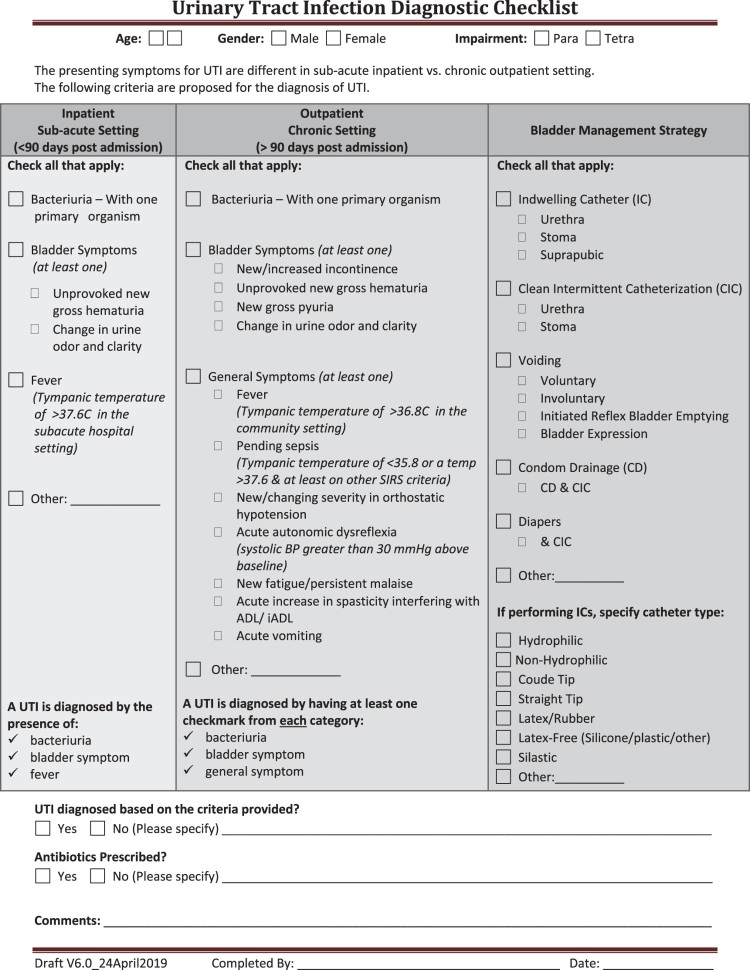
The selection and refinement of structure, process and outcome indicators related to UTI was based on the information summarized from the Driver diagram depicted in Figure 1, and the Working Group's Aim (shown below). Figure 2 displays the UTI diagnostic checklist. Embedded in this checklist is a definition of fever which reflects the current propensity for individuals with SCI/D to measure their tympanic temperature in hospital and community settings, as opposed to an oral or rectal temperature was created. Fever was defined as a tympanic temperature of ≥36.8°C in the community setting. Pending sepsis was defined as a tympanic temperature of ≤35.8°C or a temperature ≥37.6°C & at least one other SIRS criteria: Heart rate >90 bpm, Respiratory rate > 20 or PaCO2 < 32 mm Hg, WBC > 12,000/mm3, < 4,000/mm3, or > 10% bands, known source of infection.
Figure 2.
SCI-High Urinary Tract Infection Diagnostic Checklist.
Aim
The Aim of the Working Group was to reduce inappropriate antibiotic prescription for UTI in order to reduce the rising incidence of antibiotic resistance and the associated complications among individuals with chronic SCI/D living in the community. By defining UTI the Working Group intended to ensure that patients, their families and regulated health care providers recognize symptomatic UTI in order to assure a timely, and culture-specific UTI diagnosis.
Construct definition
The goals of UTI Domain are to ensure (1) patient and regulated health care provider recognition of symptomatic UTI; (2) appropriate culture-specific UTI diagnosis and timely treatment; (3) avoidance of sepsis and it's adverse impact on UTI frequency and neurorecovery; and, (4) reduce the impact of UTI on health-related quality of life.
UTI indicators
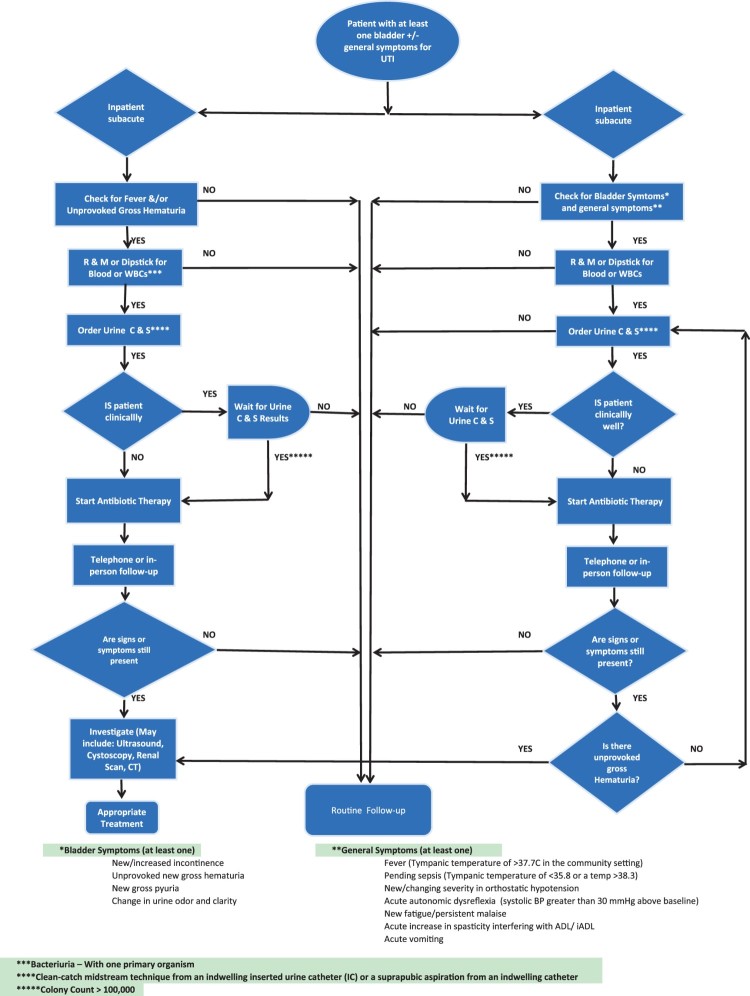
Table 1 summarizes the indicator type, denominators, and timing of measurement for each of the indicators selected by the UTI Working Group. Figure 3 displays the UTI decision tree to support practice following UTI recognition. The diagram does not specify appropriate antimicrobial therapy as this issue has been addressed in current CUA guidelines.5
Table 1. Selected structure, process and outcome indicators for the Urinary Tract Infection Domain.
| Indicator | Denominator | Type | Time of measurement |
|---|---|---|---|
| Proportion of patients with SCI/D with a health care professional (family MD, PMR/urology) Proportion of patients with SCI/D with a health care professional able to follow-up regarding urine culture and sensitivity within 48–72 h of collection |
Number of SCI/D rehabilitation admissions per fiscal year Total number of SCI/D patients who have signs or symptoms of UTI (checklist completion) |
Structure Structure |
Annual Annual |
| Proportion of SCI/D rehabilitation inpatients with UTI as defined by the UTI diagnostic checklist/definitions | Number of SCI/D rehabilitation admissions per fiscal year | Process | Rehabilitation discharge |
| Proportion of SCI/D rehabilitation inpatients with inappropriate antibiotic prescription | Number of SCI/D rehabilitation admissions per fiscal year | Outcome | Rehabilitation discharge |
Figure 3.
SCI-High Urinary Tract Infection management and treatment diagram.
Table 2 contains the structure indicator questionnaire to discern the proportion of patients with a community provided able to provide a timely review of urine culture and sensitivity results within 72 h of culture receipt. The process measure requires routine implementation of the UTI checklist for UTI diagnosis. The outcome indicator is the proportion of patients who had inappropriate antibiotic prescription.
Table 2. SCI-High Urinary Tract Infection Patient Questionnaire.
 |
Discussion
The SCI-High Domain-specific Working Group developed a framework of structure, process and outcome indicators to incrementally advance the field by 2020. The Working group chose a series of indicators to provide timely and appropriate diagnosis and treatment for UTI after SCI/D and thereby reduce antibiotic resistance among individuals with chronic SCI/D living in the community.
Individuals with an SCI/D are at increased risk of UTIs for several unique reasons: they typically require some form of instrumentation to void (potentially introducing bacteria into the bladder); they have a reduced innate immune responses in their bladder; they are frequently exposed to antibiotics which over time can select multi-drug resistant uropathogenic organisms; they are at increased risk of structural abnormalities such as vesicoureteral reflux or urinary stones; and, they may have an altered urinary microbiome.28–30 The clinical conundrum is that the one-term label of a “UTI” actually represents a heterogeneous collection of conditions ranging from asymptomatic bacteria resulting in changes in urine color/odor, infection of epididymis or testes (especially in males using transurethral catheters), symptomatic bacterial cystitis, pyelonephritis or life-threatening urosepsis.13
A crucial first step in the Working group's processes was to create a new operational definition of UTI that reflects the heterogeneity of presentation in the inpatient and outpatient settings and the challenges of diagnosing UTI among individuals in the chronic SCI/D population with multiple morbidity. First, urine cultures are often considered “positive” by health care providers due to asymptomatic bacteriuria, leading to false positive results or an inappropriate diagnosis of a UTI. Second, due to comorbid neurologic impairments, traditional UTI symptoms are often not sensitive or specific enough; this has led to a long list of potential non-specific UTI symptoms such as increased spasticity, which are confusing for patients and non-SCI specialists to interpret in isolation.31,32 Third, some of the most common symptoms that patients use in the community to determine if there is a UTI include foul-smelling or cloudy urine, both of which are common in the presence of asymptomatic bacteriuria, and in the absence of clinically relevant signs or symptoms, these individuals should not receive antibiotic treatment. Finally, routine urine testing in the absence of signs and symptoms is commonly employed in general practice, leads to the overdiagnosis of UTI.32
In the general population, UTI generally refers to symptomatic bacterial cystitis, which is a common condition that if treated without antibiotic therapy and has a low risk of progression to pyelonephritis.33 In contract, after SCI/D, there is a high rate of pyelonephritis and a higher baseline risk of urosepsis34 These facts are often a driver for over-treatment of patients in the outpatient setting with some combination of a positive urine dipstick, urine culture and/or symptoms. Non-specific UTI signs and symptoms alone perpetuate diagnostic confusion among health care providers, people living with SCI/D and their family members. The Working Group knowingly adopted a single checklist for UTI recognition, acknowledging the stark contrast in the acute hospital versus community setting's ability to monitor patients. This UTI checklist is intended for use among rehabilitation inpatients and may outpatients to facilitate routine documentation which may identify people who do not require urgent therapy.
Given the challenges with UTI recognition, timely and appropriate UTI recognition (diagnosis) and management is of utmost importance. Timely treatment is vital to mitigate urosepsis, and to reduce the associated negative impact on systemic inflammation, future UTI incidence, and functional recovery. The structure indicator was designed to quantify the availability of health care professionals to respond to patients regarding urine culture and sensitivity results within 48–72 h of collection, or to initiate therapy when cultures are pending in the presence of sepsis. It is well recognized that access to health care providers can influence patient health outcome and satisfaction.
The process indicator was selected to determine to what extent diagnosis and treatment of UTI in SCI/D population is based on the checklist as opposed to patient anxiety or physician behavior.
Antimicrobial resistance in patients with SCI/D is common and related to the widespread antibiotic use.35 Inappropriate prescription and longer duration of antibiotic therapy significantly increases antibiotic resistance. To prevent antibiotic resistance, only those patients with symptoms and signs should receive antibiotic therapy of sufficient duration.36 To address the overuse of the antibiotic for the treatment of UTI among SCI/D population, the outcome indicator was defined as the proportion of SCI/D rehabilitation inpatients who inappropriately received antibiotic therapy. Thus, a patient who does not meet the diagnostic criteria based on the UTI checklist (Figure 2) should not be treated.
The strength of the developed structure, process and outcome indicators is dependent upon their feasibility and long-term sustainability. The UTI checklist was designed as a simple tool to be used by both formal and informal care providers including family caregivers, family physicians and other specialists in the patient's care network.
Some limitations should be considered when evaluating these quality improvement indicators. First, the UTI definition does not address those SCI/D individuals with an indwelling catheter, although we do record bladder management strategy. Second, there is a lack of definition for the number of colony count for UTI in the checklist. The most frequently used threshold of for bacteriuria include>102 colony forming units (cfu)/mL as a cut-off if the urine was collected by intermittent catheterization, >104 cfu/mL for a clean void, and any detectable concentration for suprapubic aspirates.4 The number of colony-forming units has been considered in the decision tree designed by the Working Group. Third, as opposed to the EU guideline with 5 definitions for UTI, the Working Group defined a single definition for the UTI among individuals with SCI/D. Indeed, this single definition makes the diagnosis more clinically feasible for health care providers, patients and their families. Finally, the checklist designed by the Working Group requires a formal knowledge translation plan to share with community care providers. The Working Group acknowledges that using the UTI checklist is a feasible clinical definition for the most common NLUTD scenarios, but may not apply in the setting of urological procedures and will likely require further refinement.
Conclusions
These structure, process and outcome quality indicators will ultimately reduce inappropriate antibiotic therapy for UTI and the rising incidence of antibiotic resistance among community-dwelling individuals with chronic SCI/D. In future, the structure indicator will define a benchmark for timely access to health care providers for UTI management thereby promoting optimal treatment of this frequent and serious health condition among the SCI/D population.
Funding Statement
This work is embedded in the larger SCI-High Project funded by the Rick Hansen Institute (Grant #G2015-33), Ontario Neurotrauma Foundation (ONF; Grant #2018 RHI-HIGH-1057), and Toronto Rehab Foundation. Funding for open access publication fees was provided by Toronto Rehab Foundation and Coloplast A/S.
Acknowledgements
The authors would like to acknowledge the time, energy and expertise of Dr Matheus J Wiest.
Disclaimer statements
Conflict of interest Dr. Craven acknowledges support from the Toronto Rehab Foundation as the Toronto Rehabilitation Institute Chair in Spinal Cord Injury Rehabilitation and receipt of consulting fees from the Rick Hansen Institute. Dr. Karen Ethans acknowledges the receipt research grants from Rick Hansen Institute and from Ipsen Pharmaceuticals, as well as honoraria from Allergan and Ipsen. Dr. S. Mohammad Alavinia, Sandi Disher, Farnoosh Farahani, Dr. Jerzy B. Gajewski, Dr. Magdy Hassouna, Maryam Omidvar, Raj Parmar, John Shepherd, and Dr. Blayne Welk have no conflicts to disclose.
ORCID
B. Catharine Craven http://orcid.org/0000-0001-8234-6803
S. Mohammad Alavinia http://orcid.org/0000-0002-5503-9362
Maryam Omidvar http://orcid.org/0000-0003-2415-8921
Farnoosh Farahani http://orcid.org/0000-0002-3937-7708
References
- 1.Gajewski JB, Schurch B, Hamid R, Averbeck M, Sakakibara R, Agro EF, et al. . An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol Urodyn. 2018;37(3):1152–61. doi: 10.1002/nau.23397 [DOI] [PubMed] [Google Scholar]
- 2.Craven BC, Verrier M, Balioussis C, Wolfe D, Hsieh J, Noonan V, et al. . Rehabilitation environmental scan atlas: capturing capacity in Canadian SCI rehabilitation. Bladder Continence. Available from http://168.144.170.22/research/scimanifesto/D__Bladder_Continence/: Rick Hansen Institute; 2012.
- 3.Adriaansen JJ, Post MW, de Groot S, van Asbeck FW, Stolwijk-Swuste JM, Tepper M, et al. . Secondary health conditions in persons with spinal cord injury: a longitudinal study from one to five years post-discharge. J Rehabil Med. 2013;45(10):1016–22. doi: 10.2340/16501977-1207 [DOI] [PubMed] [Google Scholar]
- 4.Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, et al. . Summary of European Association of Urology (EAU) Guidelines on Neuro-urology. Eur Urol. 2016;69(2):324–33. doi: 10.1016/j.eururo.2015.07.071 [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh A, Baverstock R, Campeau L, Carlson K, Cox A, Hickling D, et al. . Canadian urological association guideline: diagnosis, management, and surveillance of neurogenic lower urinary tract dysfunction – full text. Can Urol Assoc J. 2019;13(6):E157–E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhide R, Rivers C, Kuerban D, Chen J, Farahani F, Flett H, et al. . Service interruptions and their impact on rehabilitation length of stay among Ontarians with traumatic, subacute spinal cord injury. Crit Rev Phys Rehabil Med. 2018;30:45–66. doi: 10.1615/CritRevPhysRehabilMed.2018024333 [DOI] [Google Scholar]
- 7.Guilcher SJ, Craven BC, Calzavara A, McColl MA, Jaglal SB.. Is the emergency department an appropriate substitute for primary care for persons with traumatic spinal cord injury? Spinal Cord. 2013;51(3):202–8. doi: 10.1038/sc.2012.123 [DOI] [PubMed] [Google Scholar]
- 8.Rick Hansen Spinal Cord Injury Registry – a look at traumatic spinal cord injury in Canada in 2017. Vancouver, BC: RHI; 2018. Available from http://rickhanseninstitute.org/images/stories/Article_PDFs/SCI_Report_dec9_web1.pdf.
- 9.Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings SE, Wagenlehner F, et al. . EAU Guidelines on Urological Infections: European Association of Urology 2017.
- 10.Brommer B, Engel O, Kopp MA, Watzlawick R, Muller S, Pruss H, et al. . Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain. 2016;139(Pt 3):692-707. doi: 10.1093/brain/awv375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp MA, Watzlawick R, Martus P, Failli V, Finkenstaedt FW, Chen Y, et al. . Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology. 2017;88(9):892–900. doi: 10.1212/WNL.0000000000003652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biering-Sorensen F, Bagi P, Hoiby N.. Urinary tract infections in patients with spinal cord lesions: treatment and prevention. Drugs. 2001;61(9):1275–87. doi: 10.2165/00003495-200161090-00004 [DOI] [PubMed] [Google Scholar]
- 13.Alavinia SM, Omidvar M, Farahani F, Bayley M, Zee J, Craven BC.. Enhancing quality practice for prevention and diagnosis of urinary tract infection during inpatient spinal cord rehabilitation. J Spinal Cord Med. 2017;40(6):803–12. doi: 10.1080/10790268.2017.1369216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyndaele JJ. Intermittent catheterization: which is the optimal technique? Spinal Cord. 2002;40:432–7. doi: 10.1038/sj.sc.3101312 [DOI] [PubMed] [Google Scholar]
- 15.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. . Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–63. doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 16.Dow G, Rao P, Harding G, Brunka J, Kennedy J, Alfa M, et al. . A prospective, randomized trial of 3 or 14 days of ciprofloxacin treatment for acute urinary tract infection in patients with spinal cord injury. Clin Infect Dis. 2004;39(5):658–64. doi: 10.1086/423000 [DOI] [PubMed] [Google Scholar]
- 17.Dellit TH, Owens RC, McGowan JE Jr., Gerding DN, Weinstein RA, Burke JP, et al. . Infectious Diseases Society of America and the Society for healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77. doi: 10.1086/510393 [DOI] [PubMed] [Google Scholar]
- 18.Skelton F, Suda K, Evans C, Trautner B.. Effective antibiotic stewardship in spinal cord injury: challenges and a way forward. J Spinal Cord Med. 2019;42(2):251–4. doi: 10.1080/10790268.2017.1396183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell SM, Braspenning J, Hutchinson A, Marshall MN.. Research methods used in developing and applying quality indicators in primary care. Br Med J. 2003;326(7393):816–9. doi: 10.1136/bmj.326.7393.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idvall E, Rooke L, Hamrin E.. Quality indicators in clinical nursing: a review of the literature. J Adv Nurs. 1997;25(1):6–17. doi: 10.1046/j.1365-2648.1997.1997025006.x [DOI] [PubMed] [Google Scholar]
- 21.Selim AJ, Berlowitz DR, Fincke G, Rosen AK, Ren XS, Christiansen CL, et al. . Risk-adjusted mortality rates as a potential outcome indicator for outpatient quality assessments. Med Care. 2002;40(3):237–45. doi: 10.1097/00005650-200203000-00007 [DOI] [PubMed] [Google Scholar]
- 22.Alavinia SM, Hitzig SL, Farahani F, Flett H, Bayley M, Craven BC.. Prioritization of rehabilitation domains for establishing spinal cord injury high performance indicators using a modification of the Hanlon method: SCI-High Project. J Spinal Cord Med. 2019;42(Suppl 1):S43–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craven BC, Alavinia SM, Wiest M, Farahani F, Hitzig SL, Flett H, et al. . Methods for development of structure, process and outcome indicators for prioritized spinal cord injury rehabilitation domains: SCI-High Project. J Spinal Cord Med. 2019;42(Suppl 1):S51–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mainz J. Developing evidence-based clinical indicators: a state of the art methods primer. Int J Qual Health Care. 2003;15(Suppl 1):5i–11. doi: 10.1093/intqhc/mzg084 [DOI] [PubMed] [Google Scholar]
- 25.Kavanagh A, Baverstock R, Campeau L, Carlson K, Cox A, Hickling D, et al. . Canadian Urological Association guideline: Diagnosis, management, and surveillance of neurogenic lower urinary tract dysfunction - Full text. Can Urol Assoc J. 2019;13(6):E157–E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welk B. The Canadian Spinal Cord Injury Urohealth Summit, Final report 2017. Available from http://onf.org/system/attachments/476/original/SCI_Urohealth_Summit.pdf.
- 27.Scovil CY, Delparte JJ, Walia S, Flett HM, Guy SD, Wallace M, et al. . Implementation of pressure injury prevention best practices across 6 Canadian rehabilitation Sites: results from the spinal cord injury knowledge Mobilization Network. Arch Phys Med Rehabil. 2019;100(2):327–35. doi: 10.1016/j.apmr.2018.07.444 [DOI] [PubMed] [Google Scholar]
- 28.Chaudhry R, Madden-Fuentes RJ, Ortiz TK, Balsara Z, Tang Y, Nseyo U, et al. . Inflammatory response to Escherichia coli urinary tract infection in the neurogenic bladder of the spinal cord injured host. J Urol. 2014;191(5):1454–61. doi: 10.1016/j.juro.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 29.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, et al. . Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:174. doi: 10.1186/1479-5876-10-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigil HR, Hickling DR.. Urinary tract infection in the neurogenic bladder. Transl Androl Urol. 2016;5(1):72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz LL, Cardenas DD, Kennelly M, Bonne Lee BS, Linsenmeyer T, Moser C, et al. . International spinal cord injury urinary tract infection basic data set. Spinal Cord. 2013;51(9):700–4. doi: 10.1038/sc.2013.72 [DOI] [PubMed] [Google Scholar]
- 32.Skelton F, Grigoryan L, Holmes SA, Poon IO, Trautner B.. Routine urine testing at the spinal cord injury annual evaluation leads to unnecessary antibiotic use: a pilot study and future directions. Arch Phys Med Rehabil. 2018;99(2):219–25. doi: 10.1016/j.apmr.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gágyor I, Bleidorn J, Kochen M, Schmiemann G, Wegscheider K, Hummers-Pradier E.. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial2015. h6544 p. [DOI] [PMC free article] [PubMed]
- 34.Welk B, Liu K, Winick-Ng J, Shariff SZ.. Urinary tract infections, urologic surgery, and renal dysfunction in a contemporary cohort of traumatic spinal cord injured patients. Neurourol Urodyn. 2017;36(3):640–7. doi: 10.1002/nau.22981 [DOI] [PubMed] [Google Scholar]
- 35.Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA.. Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch Phys Med Rehabil. 2000;81(6):764–9. doi: 10.1016/S0003-9993(00)90108-4 [DOI] [PubMed] [Google Scholar]
- 36.Garcia Leoni ME, Esclarin De Ruz A.. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9(8):780–5. doi: 10.1046/j.1469-0691.2003.00643.x [DOI] [PubMed] [Google Scholar]