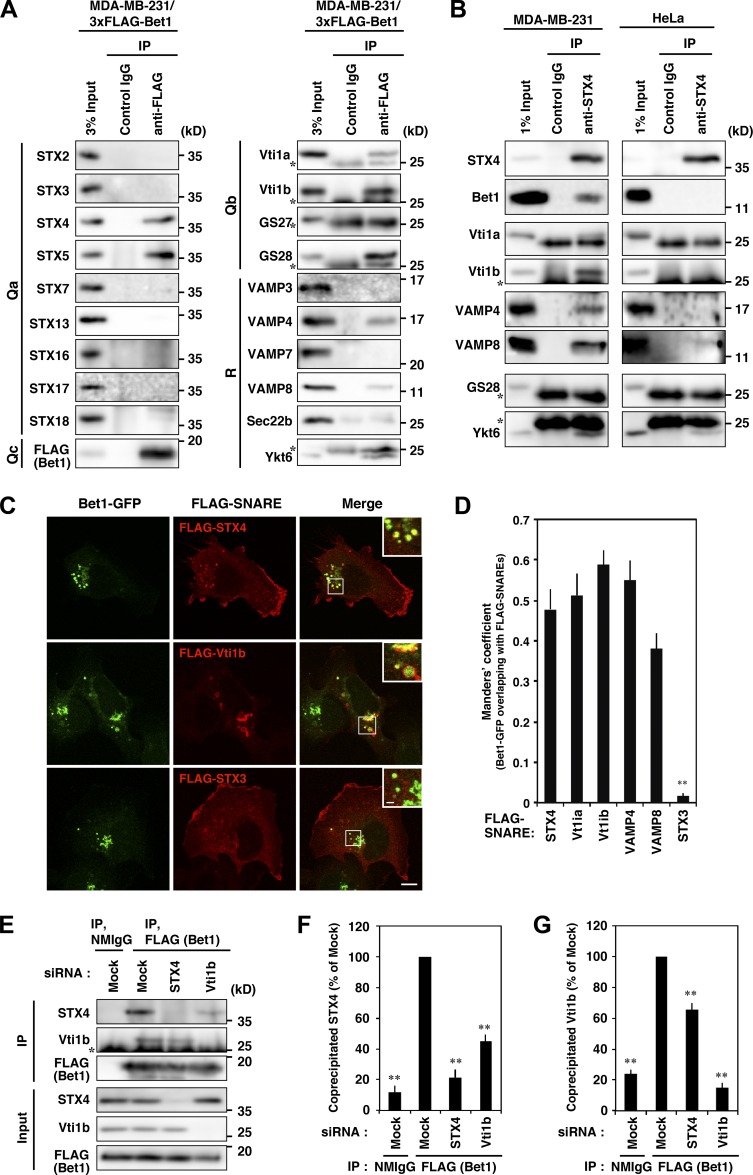
Figure 5.
Bet1 forms novel SNARE complexes with endosomal SNAREs in MDA-MB-231 cells. (A) 3xFLAG-Bet1 interacts with STX4, Vti1b, and VAMP4 in addition to STX5. MDA-MB-231 cells stably expressing 3xFLAG-Bet1 were lysed, and the lysate was subjected to immunoprecipitation using FLAG-beads, and then the precipitate was subjected to immunoblotting for endogenous SNARE proteins. In the following blots, the brightness and contrast were adjusted so that their input bands became comparable to those of other SNAREs: STX3, STX7, STX16, STX17, STX18, VAMP3, VAMP7, and Vti1b. (B) An endogenous complex comprised of STX4, Bet1, Vti1b, and VAMP4 exists in MDA-MB-231 cells, but not in HeLa cells. Lysates of MDA-MB-231 and HeLa cells were subjected to immunoprecipitation of STX4, and then the co-precipitation of Bet1, Vti1b, and VAMP4 was analyzed. In the following blots, the brightness and contrast were adjusted so that their input bands became comparable to those of other SNAREs: Bet1 and VAMP4 in MDA-MB-231 cells and Bet1, VAMP4, and VAMP8 in HeLa cells. (C and D) Bet1-interacting SNAREs are colocalized with Bet1-GFP in endomembrane compartments. MDA-MB-231 cells stably expressing Bet1-GFP were transfected with FLAG-SNAREs and then subjected to immunofluorescence and confocal microscopy (C). Their colocalization was assessed by using the Manders’ overlap coefficient (D). (E–G) Depletion of STX4 and Vti1b reduces the complex of Bet1 with Vti1b and STX4, respectively. MDA-MB-231 cells stably expressing 3xFLAG-Bet1 were transfected with STX4 and Vti1b siRNAs, and then immunoprecipitation and immunoblotting (E) were performed as in A. Co-precipitated endogenous STX4 (F) and Vti1b (G) were quantified. Scale bar: 10 μm in a regular image; 2 μm in an inset. **, P < 0.01; vs. STX4 in D; vs. FLAG (Bet1), mock in F and G.