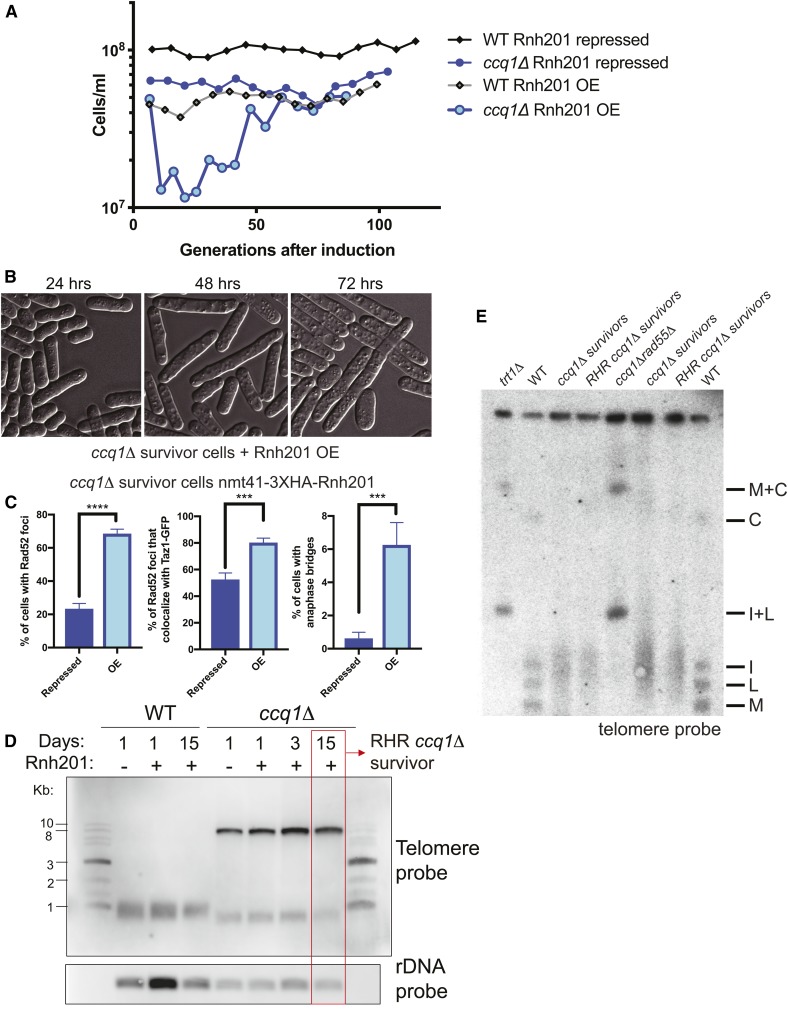
Figure 3.
Degradation of RNA–DNA hybrids induces a second growth crisis in ccq1Δ survivors, ultimately leading to a new type of RNase H-resistant ccq1Δ survivor. (A) Rnh201 induction returns ccq1Δ survivors into a growth crisis. WT or ccq1Δ Taz1-GFP survivor cells (MKSP1213 and MKSP1214) were modified to insert the nmt41+ promoter upstream of the rnh201+ coding sequence. Strains were then cultured in a repressed state (with thiamine) or induced state (without thiamine), as indicated and diluted every 24 hr (B) DIC imaging shows that Rnh201 overexpression leads to a new growth crisis in ccq1Δ survivors. The first 3 days of liquid culturing corresponding to (A) are shown. Note the increase in cell length, indicating checkpoint arrest. (C) Rnh201 overexpression (OE) leads to increased Rad52-mCherry loading at telomeres and anaphase bridges. (D and E) The telomeres of RHR ccq1Δ survivors selected after constitutive Rnh201 overexpression are similar in length and structure to the initial ccq1Δ survivors. (C) Southern blot of EcoRI-digested genomic DNA as in Figure S1B. The rDNA probe serves as a loading control. (E) The RHR ccq1Δ survivors, like ccq1Δ survivors, retain linear chromosomes as assessed by pulsed-field gel electrophoresis followed by Southern blotting. Genomic DNA was digested with NotI and hybridized to C, I, L, and M probes, which detect the terminal fragments of chromosomes I and II. Bands corresponding to chromosome end fusions are indicated by M+C and I+L bands, observed in the trt1Δ and ccq1Δrad55Δ circular survivors.