Abstract
We report a case of a 60-year-old man who was a former cigar smoker with a slow-growing, large exophytic left shoulder mass (15 cm in diameter) and later found to have left axillary lymphadenopathy. Fine needle aspirate biopsy of the left shoulder mass revealed squamous cell carcinoma (SCC). However, pathology of the enlarged left axillary lymph node was reported as metastatic adenocarcinoma. The patient underwent surgical resection of the shoulder mass which comprised of SCC (>95%) and adenoid basal cell carcinoma (BCC) as a second component of the tumour. The BCC had identical histology as the metastatic carcinoma in the left axillary lymph node. Therefore, diagnosis was revised as cutaneous collision tumour with metastatic BCC. Six months later following adjuvant radiation therapy, the patient was diagnosed with metastatic BCC in the right lung. Stereotactic body radiation therapy (SBRT) and a selective hedgehog pathway inhibitor vismodegib were given with only limited efficacy. Clinical trial registration number NCT03132636.
Keywords: lung cancer (oncology), skin cancer
Background
Collision tumours are rare neoplastic occurrences where at least two independent neoplasms develop in proximity to each other. In an analysis of 69 cases of cutaneous collision tumours, basal cell carcinoma (BCC) containing a melanocytic nevus (14/69) is the most common combination.1 Skin collision tumours can also occur with neurofibromas, trichoepitheliomas and fibroxanthomas.1 Collision tumours comprised of BCC and squamous cell carcinoma (SCC) are rare and only have been reported recently.2 3 While BCCs can be locally aggressive, they rarely metastasise to distant sites such as the axillary lymph nodes, lungs and bones, as SCCs do.4 We report a collision tumour with an aggressive adenoid BCC that metastasised to the axillary lymph node and lung.
Case presentation
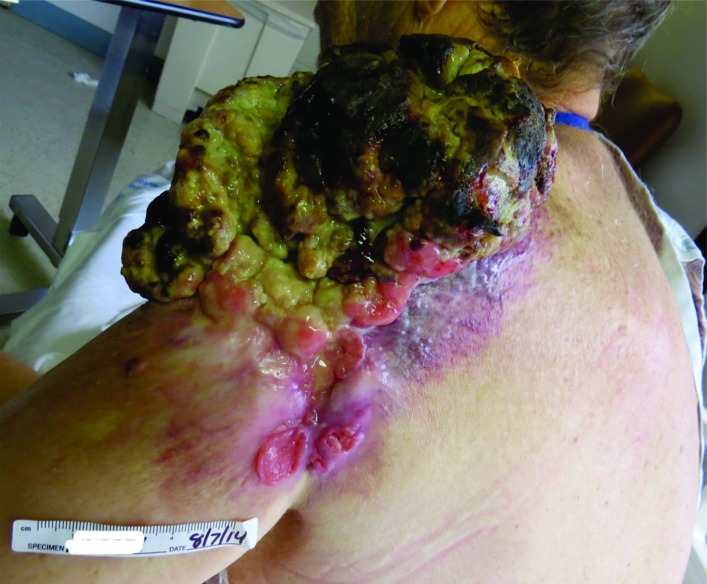
A 60-year-old man presented to his primary care physician for a non-painful, fungating mass of his left shoulder that had been slowly growing over 10 years and had recently become malodorous and bloody (figure 1). The patient was also complaining of 2 months of fatigue and difficulty breathing. His temperature was 37.8°C and his blood pressure was 84/56 mm Hg with a pulse of 111. Physical examination revealed a large, exophytic, necrotic 15×15 cm mass of the left shoulder with some mild erythema but was non-tender to palpation. The patient was given intravenous fluids and transferred to the emergency department for evaluation and management.
Figure 1.
Left shoulder mass,
Investigations
Laboratory results showed a leucocyte count of 22x109/L (4.5–11) and an absolute neutrophil count of 18 700/uL (1100–7700), haemoglobin of 87 g/L (133–177), BUN 19 mg/dL (5–25 mg/dL), creatinine of 1.51 mg/dL and a lactate of 4.2 mmol/L (0.5–2.2). The patient was admitted to the hospital and a general surgery team was consulted.
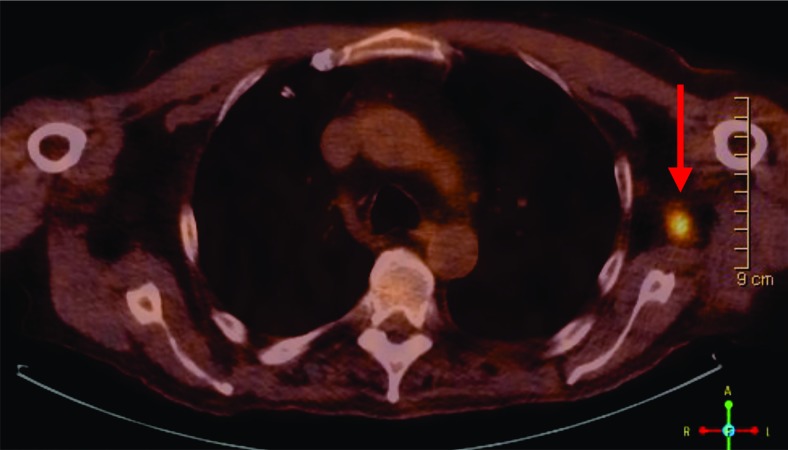
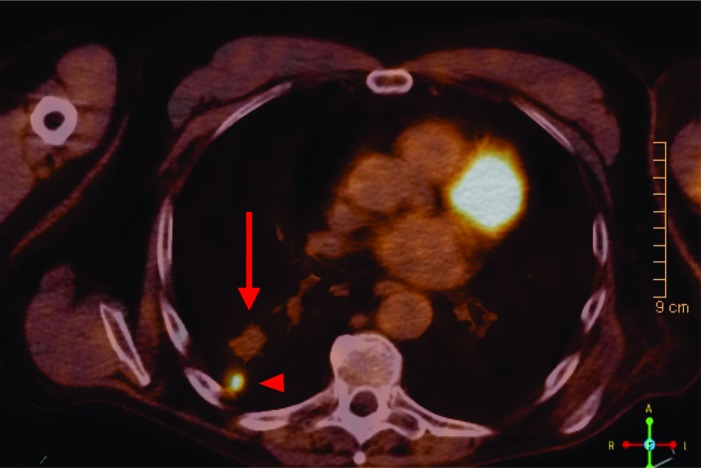
An MRI of the shoulder revealed a large fungating, enhancing 18×14×9 cm mass arising from the deltoid subcutaneous tissues but no definite osseous invasion. A positron emission tomography-computed tomography (PET/CT) performed without contrast demonstrated an intensely hypermetabolic with a standardized uptake value(SUV) of 20.6, large exophytic left shoulder soft tissue mass probably invading the deltoid region. There was also a 4.6×2.2 cm left axillary lymph node with moderate to intense fluorodeoxyglucose (FDG) uptake (maximum SUV 4.8) and a more superior 1.2 cm left axillary node with intense uptake (figure 2). There was a 6 mm nodule in the right lower lobe too small for evaluation by PET.
Figure 2.
PET/CT showing one left axillary lymph node (arrow) with intense FDG uptake.
The patient underwent a fine needle aspirate of the left shoulder mass and the pathology was reported as a poorly differentiated carcinoma with an immunohistochemical stains positive for p63 and pan-cytokeratin, compatible with SCC. Later, he underwent a left axillary lymph node core biopsy which was reported as metastatic adenocarcinoma with some features of adenoid cystic carcinoma. At that time, the pathologists could not rule out cutaneous origin of the lymph node metastasis and recommended a larger biopsy of the shoulder and the enlarged axillary lymph node for pathological comparisons.
Treatment
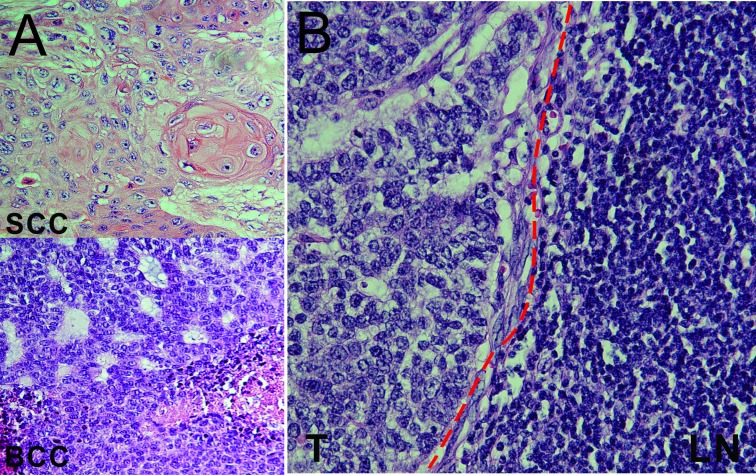
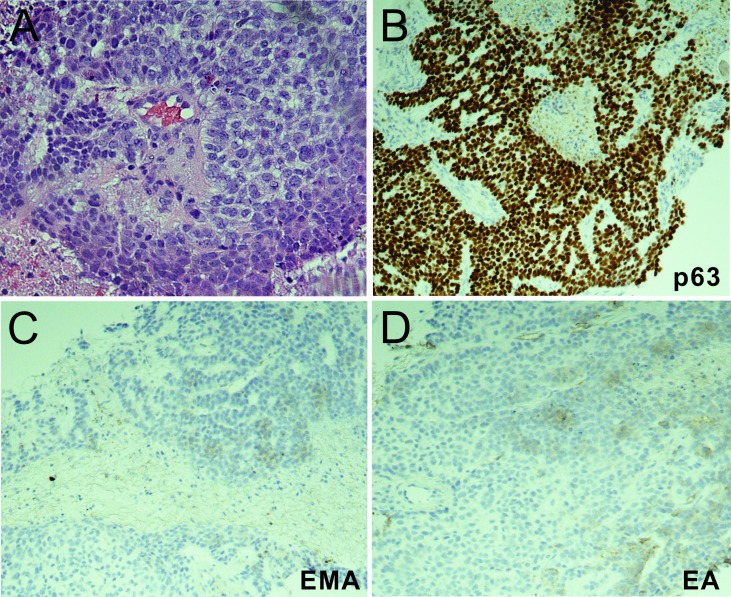
The patient had surgical resection of the left shoulder mass and the enlarged left lymph node with latissimus dorsi flap closure of the shoulder wound. The pathology report revealed that the shoulder mass was a cutaneous collision tumour containing predominantly (>95%) SCC and a second component of adenoid BCC (figure 3A) while the left lymph node only contained adenoid BCC (figure 3B). He was referred to radiation oncology and completed adjuvant radiation treatments to the left shoulder tumour bed and axilla. Six months later, the right lung nodule had grown from 6 mm to 9 mm during surveillance and biopsy of the lung nodule revealed metastatic adenoid BCC (figure 4A–D) for which he was treated with stereotactic body radiation therapy. However, 6 months later, a PET/CT scan showed increased size of the left axillary lymph node mass and of the previously treated right lower lobe lung nodule, which were concerning for disease progression. Therefore, the patient was treated with hedgehog inhibitor vismodegib for approximately 15 months but had stopped it when a PET/CT scan showed a progressively enlarging left axillary mass, a modestly increasing in size of the right lower lobe lung nodule and a new satellite nodule with avid FDG uptake (figure 5). Biopsy of the new satellite nodule revealed poorly differentiated SCC. The patient is currently on cemiplimab, an antiprogram death-1 (PD-1) inhibitor, to treat the metastatic SCC.
Figure 3.
(A) Shoulder mass excision pathology demonstrates predominantly (>95%) squamous cell carcinoma (SCC) and adenoid basal cell carcinoma (BCC), H&E staining (400×). (B) H&E staining from a resected left axillary lymph node (LN) showing metastatic adenoid BCC. The dash line separates the tumour from the lymphoid tissue (400×).
Figure 4.
(A) H&E staining of the metastatic lung adenoid basal cell carcinoma from needle biopsy (400×). (B–D) Immunohistochemistry staining of the metastatic lung lesion, which is positive for p63 (B), and negative for epithelial membrane antigen (EMA) (C) and epithelial antigen (EA) (D).
Figure 5.
PET/CT showing the previous metastatic right lung nodule (arrow) and a new satellite nodule which has active FDG update (arrowhead).
Outcome and follow-up
One year after the patient’s last radiation therapy treatment to his left axillary tumour, the patient continues to have good exercise tolerance and his only major symptom is left arm lymphedema. A PET/CT scan showed the left axillary mass is stable but with findings suggestive of central necrosis.
Discussion
BCC, the most common skin cancer, may progress locally, but rarely metastasises.4 5 BCC has several histological subtypes including superficial, nodulocystic, adenoid, pigmented, micronodular, fibroepithelioma of Pinkus, metatypical (basosquamous) and morphea-form.6–8 Adenoid BCC accounts for about 10% among all histopathological subtypes of BCCs and often occurs as a pure-form or in association with the nodular subtype. It is no more aggressive than nodular BCCs although invading behaviours such as perivascular and perineural infiltration have been reported.8–10 Histologically, we observed extensive necrosis and a large number of inflammatory cells in the collision tumour, leading us to hypothesise that the predominant SCC component may provide an inflammatory microenvironment to promote development and metastasis of the adenoid BCC. Further proof of our hypothesis requires more experimental investigations.
Identifying lung or lymph node metastatic lesions with adenocarcinoma features from an unknown primary site is often a challenge for clinicians.11 In these situations, adenoid BCC may be considered in the list of differential diagnosis. In addition, given a primary cutaneous tumour and a metastatic lesion with different histology, multiple biopsies of the primary tumour may reveal a second malignant component that is responsible for metastasis.
To treat metastatic BCC, the hedgehog inhibitors, vismodegib or sonidegib, are commonly used.4 12 13 Although the objective response rate seems to be lower in metastatic BCC than local advanced BCC in several studies, there is no data suggesting adenoid BCC is more resistant to hedgehog inhibitors than other subtypes.14 15 In the present case, the metastatic adenoid BCC progressed despite vismodegib treatment. It is unclear whether this is due to primary resistance or acquired resistance considering potential interactions between the BCC and SCC component of the collision tumour in this patient.16 17 To treat hedgehog inhibitor resistant tumours, immunotherapy is currently under investigation. There have been several case reports showing remarkable and sustained antitumour response with PD-1/PD-L1 blockade regardless of PD-L1 expression in vismodegib resistant BCC tumours.18 19
To our knowledge, this is the first case reporting the lung and axillary lymph node metastasis from adenoid BCC component of a skin collision tumour. While SCC is well-known for its metastatic potential, BCC rarely metastasises. In the current case, the BCC component took account only <5% and the SCC >95% of this collision tumour. However, the metastatic component of the lung and lymph node is composed of 100% of adenoid BCC. Because BCCs rarely metastasise, accurate diagnosis of potential primary site causes substantial difficulties by both original pathologist and expert consultants. In addition, whether the resistance to vismodegib in this patient is associated with the collision tumour is open to discussion. Whether the prognosis of patients with malignant skin collision tumour such as SCC/adenoid BCC is different from patients with isolated forms of skin cancer is also unknown. Overall, the diagnosis, treatment and prognosis of metastatic collision tumours is a new and unmet clinical need required more studies and attentions.
Learning points.
This is a rare case of skin collision tumour comprising of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).
Metastasis from BCC can occur in the context of collision tumour even when BCC is the minor component of the tumour, as in this case, BCC was <5%.
Biopsy of two or more sites of the primary cutaneous tumour may aid diagnosis if the primary and metastatic tumours reveal inconsistent histology.
The potential interactions between SCC and BCC in the collision tumour may alter disease progression, response to treatment and patient prognosis of isolated SCC or BCC.
Footnotes
Contributors: RL and GL wrote the case report. MH provided intellectual input for the discussion. MH and AE-S provided radiology and pathology images. RL and GL finalised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Boyd AS, Rapini RP. Cutaneous collision tumors. An analysis of 69 cases and review of the literature. Am J Dermatopathol 1994;16:253–7. [PubMed] [Google Scholar]
- 2. Lam C, Fuller C, Flamm A, et al. . Collision tumor of basal and squamous cell carcinoma of the palm. J Clin Aesthet Dermatol 2019;12:28–30. [PMC free article] [PubMed] [Google Scholar]
- 3. Sterz H, Kendler M, Simon J-C, et al. . [Malignant collision tumor of the skin : Melanoma, squamous cell carcinoma and basal cell carcinoma]. Pathologe 2019;40:534–8. 10.1007/s00292-019-0622-3 [DOI] [PubMed] [Google Scholar]
- 4. Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med 2018;379:363–74. 10.1056/NEJMra1708701 [DOI] [PubMed] [Google Scholar]
- 5. Moser S, Borm J, Mihic-Probst D, et al. . Metastatic basal cell carcinoma: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:e79–82. 10.1016/j.oooo.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 6. Stoica LE, Georgescu CV, Pătraşcu V, et al. . Basal cell carcinomas - clinical-evolutional and histopahotologic aspects. Curr Health Sci J 2009;35:228–33. [PMC free article] [PubMed] [Google Scholar]
- 7. Koyuncuer A. Histopathological evaluation of non-melanoma skin cancer. World J Surg Oncol 2014;12:159 10.1186/1477-7819-12-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mackiewicz-Wysocka M, Bowszyc-Dmochowska M, Strzelecka-Weklar D, et al. . Basal cell carcinoma – diagnosis. Contemp Oncol 2013;17:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saxena K, Manohar V, Bhakhar V, et al. . Adenoid basal cell carcinoma: a rare facet of basal cell carcinoma. BMJ Case Rep 2016;2016:bcr2015214166 10.1136/bcr-2015-214166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jetley S, Jairajpuri ZS, Rana S, et al. . Adenoid basal cell carcinoma and its mimics. Indian J Dermatol 2013;58:244 10.4103/0019-5154.110874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fizazi K, Greco FA, Pavlidis N, et al. . Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26(Suppl 5):v133–8. 10.1093/annonc/mdv305 [DOI] [PubMed] [Google Scholar]
- 12. Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol 2018;79:1089–100. 10.1016/j.jaad.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 13. Jacobsen AA, Aldahan AS, Hughes OB, et al. . Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies. JAMA Dermatol 2016;152:816–24. 10.1001/jamadermatol.2016.0780 [DOI] [PubMed] [Google Scholar]
- 14. Basset-Seguin N, Hauschild A, Grob J-J, et al. . Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol 2015;16:729–36. 10.1016/S1470-2045(15)70198-1 [DOI] [PubMed] [Google Scholar]
- 15. Sekulic A, Migden MR, Lewis K, et al. . Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol 2015;72:1021–6. 10.1016/j.jaad.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 16. Ransohoff KJ, Tang JY, Sarin KY. Squamous change in basal-cell carcinoma with drug resistance. N Engl J Med 2015;373:1079–82. 10.1056/NEJMc1504261 [DOI] [PubMed] [Google Scholar]
- 17. Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol 2014;150:970–3. 10.1001/jamadermatol.2014.583 [DOI] [PubMed] [Google Scholar]
- 18. Falchook GS, Leidner R, Stankevich E, et al. . Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer 2016;4 10.1186/s40425-016-0176-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischer S, Ali OH, Jochum W, et al. . Anti-Pd-1 therapy leads to Near-Complete remission in a patient with metastatic basal cell carcinoma. Oncol Res Treat 2018;41:391–4. 10.1159/000487084 [DOI] [PubMed] [Google Scholar]