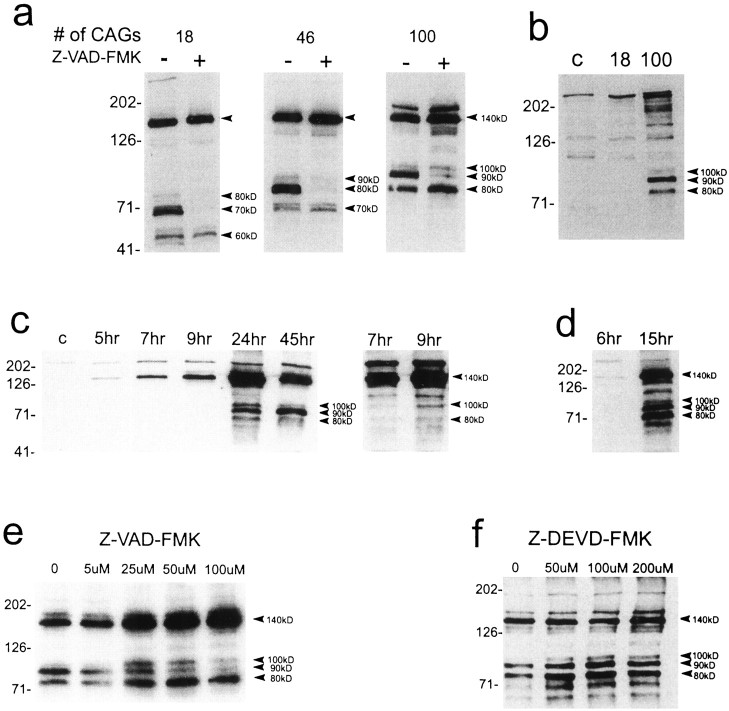
Fig. 8.
Western blots of huntingtin expression in protein extracts of transfected striatal cells and the effects of caspase inhibitors. a, Human huntingtin is seen at the expected size of ∼140 kDa (top arrowhead) in cells exposed to the FH3221 expression plasmids. A slightly larger band at ∼175 kDa is also seen with FH3221-100. N-terminal products (bottom arrowheads) occur with wild-type (18 CAGs) and mutant (46 and 100 CAGs) huntingtins and show variable size consistent with polyglutamine expansion. Native huntingtin appears at the top of first lane. Z-VAD-FMK (100 μm) markedly inhibits a prominent intermediate N-terminal product in wild-type huntingtin (70 kDa) and mutant huntingtins (80 kDa for 46 CAGs and 90 kDa for 100 CAGs). b, Expression of full-length wild-type huntingtin (18 CAGs) and mutant huntingtin (100 CAGs) with FH9774 constructs generates N-terminal products (arrowheads) only from mutant huntingtin. Lane c was nontransfected and shows native mouse huntingtin at thetop of the blot. Full-length human mutant huntingtin migrates above native mouse protein. c,d, Time course of appearance of N-terminal products after expression of FH3221-100. Results in cand d are from different transfections. The blot at theright in c is a longer exposure of the 7 and 9 hr time points. The 140 kDa band appears at 5 and 6 hr. The 100 kDa product is seen at 9 hr (c, right blot). All three N-terminal products are present at 15 hr (d). The 90 kDa band is the last to appear at 15 hr (d) and the most prominent at 45 hr (c). Native huntingtin appears at thetop of most lanes (c).e, Effects of Z-VAD-FMK concentration (0–100 μm) on mutant huntingtin expressed from FH3221-100. The 90 kDa N-terminal product is attenuated at all concentrations and maximally at 100 μm. Increased expression of the 140, 80, and 100 kDa proteins are seen and may be related to increased viability. f, Effects of concentration of Z-DEVD-FMK (0–200 μm) on mutant huntingtin expression by FH3221-100. No inhibitory effects on cleavage are seen at any concentration. N-terminal products are slightly elevated, but the 140 kDa protein is unchanged in cells treated with the inhibitor. Protein extracts (20 μg/lane) were taken 24 hr after transfection in a, b,e, and f. Antibody Ab 1 was used to detect huntingtin. Molecular mass markers are shown to theleft of each blot.