Abstract
Sequence database searches with the α2δ subunit as probe led to the identification of two new genes encoding proteins with the essential properties of this calcium channel subunit. Primary structure comparisons revealed that the novel α2δ-2 and α2δ-3 subunits share 55.6 and 30.3% identity with the α2δ-1 subunit, respectively. The number of putative glycosylation sites and cysteine residues, hydropathicity profiles, and electrophysiological character of the α2δ-3 subunit indicates that these proteins are functional calcium channel subunits. Coexpression of α2δ-3 with α1C and cardiac β2a or α1E and β3 subunits shifted the voltage dependence of channel activation and inactivation in a hyperpolarizing direction and accelerated the kinetics of current inactivation. The kinetics of current activation were altered only when α2δ-1 or α2δ-3 was expressed with α1C. The effects of α2δ-3 on α1C but not α1E are indistinguishable from the effects of α2δ-1. Using Northern blot analysis, it was shown that α2δ-3 is expressed exclusively in brain, whereas α2δ-2 is found in several tissues. In situ hybridization of mouse brain sections showed mRNA expression of α2δ-1 and α2δ-3 in the hippocampus, cerebellum, and cortex, with α2δ-1 strongly detected in the olfactory bulb and α2δ-3 in the caudate putamen.
Keywords: α2δ subunit, voltage-activated channel, calcium channel subunit, neuron, gene diversity, electrophysiology
Voltage-gated calcium channels have been purified and cloned from various tissues such as skeletal muscle, heart, and brain. These channels are formed by heterooligomeric complexes consisting of various combinations of an α1protein with auxiliary α2δ, β, and γ subunits. To date, seven genes encoding α1 subunits of the high voltage-activated (for review, see Hofmann et al., 1994; Strom et al., 1998; Bech-Hansen et al., 1998) and two genes of the low voltage-activated calcium channels have been identified (Perez-Reyes et al., 1998; Cribbs et al., 1998). This subunit accounts not only for the ion channel pore but also contains the voltage sensor and the determinants for binding of drugs and toxins. The current through the α1 subunit is modulated by interactions with the β, α2δ, and γ subunits. The molecular diversity of the β subunit is not only caused by the expression of four genes, but also by differential splicing. Until recently, only a single γ subunit in skeletal muscle had been described (Eberst et al., 1997), but a novel neuronal form has since been identified in brain (Letts et al., 1998).
Since the molecular cloning of the α2δ subunit (Ellis et al., 1988), several splice variants have been detected, but no further α2δ subunit genes have been identified. The different splice variants arise from various combinations of three alternatively spliced regions that result in five isoforms that are expressed in a tissue-specific manner (Angelotti and Hofmann, 1996). Structurally, the α2δ subunit is a heavily glycosylated 175 kDa protein that is posttranslationally cleaved to yield a disulfide-linked α2 and δ protein (DeJongh et al., 1990; Jay et al., 1991). The δ part anchors the α2protein to the membrane via a single transmembrane segment (Gurnett et al., 1996). The membrane topology of the α2δ subunit was further refined using anti-α2 antibodies and C-terminal deletion mutants (Brickley et al., 1995; Wiser at al., 1996). Structural studies have shown that the extracellular α2 domain provides the structural elements required for channel stimulation (Gurnett et al., 1996) and that the δ domain, which contains the only transmembrane segment of α2δ complex, harbors the regions important for the shift in voltage-dependent activation, steady-state inactivation, and the modulation of the inactivation kinetics (Felix et al., 1997). The identification of new α2δ subunits could present further possibilities for differential and specific regulation of calcium channels.
In studies designed to address the diversity and function of α2δ, cloning, hybridization, and patch-clamp techniques were used to identify and characterize novel α2δ subunits. Two new α2δ genes were found by searching databases with the α2δ cDNA sequence (Ellis et al., 1988). These genes encode proteins with essential features of α2δ subunits, such as the high number of potential glycosylation sites and cysteine residues, the hydrophobicity plots, and electrophysiological characteristics. The novel α2δ-2 gene was found to be predominately expressed in heart, pancreas, and skeletal muscle, with α2δ-3 expressed only in brain.
MATERIALS AND METHODS
Isolation of RNA and cDNA library construction. Total RNA from mouse brain was isolated by the guanidinium thiocyanate method, and the poly(A) RNA was separated by oligo-dT cellulose chromatography (Poly(A) Quik mRNA Isolation kit; Stratagene, Heidelberg, Germany). Poly(A) RNA from mouse brain was reverse-transcribed, and double-strand cDNA was synthesized using the Superscript plasmid system (Life Technologies). The cDNA fragments were ligated with BstXI/EcoRI adaptors (Invitrogen, San Diego, CA) and size-fractionated in a low-melting agarose gel. Only gel slices containing fragments >2000 bp were excised and digested with Gelase (Biozym, Hessisch Oldendorf, Germany). Recovered cDNA was ligated into the BstXI site of the pcDNAII vector (Invitrogen) and transformed in the Escherichia coliXL1-blue mrf′ strain (Stratagene, Heidelberg, Germany). The cDNA library was screened with a random-primed labeled PCR probe (see below, nt 1501–1821). Sequencing of the clones was performed using the dideoxy chain termination method on both strands. A full-length clone was used for the preparation of the NotI-SphI fragment with the entire open reading frame in pcDNA3 (Invitrogen) yielding the expression plasmid pc3α2δ-3.
PCR amplification. The expressed sequence tag (EST) with the accession number AA190607 was used to design the primers NKAD1, GGC ACA GAT GTC CCA GTT AAA GA and NKAD2, TGT ATA GTA GTA GTC ATT GGT CAT, with which the partial α2δ-3 subunit cDNA was amplified. The PCR fragment was cloned in pUC18 and was sequenced on both strands.
Northern blot analysis. Human and mouse multiple tissue Northern Blots were obtained from Clontech (Heidelberg, Germany) and hybridized according to the manufacturer’s instructions. Random-primed labeled fragments (α2δ-2, nt 2877–3249; α2δ-3, nt 2893–3377) were used as probes; these regions share no significant homology with each other. A 3 hr prehybridization step was followed by an overnight hybridization with 5 × 106 cpm/ml of probe at 42°C. The final stringency wash performed was with 0.1× SSC, 0.1% SDS at 42°C.
In situ hybridization. Intact brains of adult female mice were removed immediately after cervical dislocation and frozen in isopentane cooled to −40°C. Brain was sectioned into 16 μm slices in a cryostat at −20°C and thaw-mounted onto polylysine slides. The tissue was vacuum-dried, fixed in 4% paraformaldehyde in PBS, and washed in 0.5× SSC. The sections were acetylated in 0.25% acetanhydride in 0.1 m triethanolamine, pH 8.0, and washed in 2× SSC. The tissue was dehydrated through a series of ethanol solutions, from 50 to 100%. The slides were vacuum-dried and stored at −80°C until used.
Murine α2δ-1 (nt 2760–3170), human α2δ-2 (nt 2877–3249), and murine α2δ-3 (nt 2744–3228) specific riboprobes were generated by in vitro transcription as described previously (Ludwig et al., 1997). Briefly, probe template DNA was amplified by PCR from murine α2δ-1 and -2 clones using primers specific for the variable C-terminal region of α2. BamHI andAsp718 restriction sites were added to the α2δ-1 forward and reverse primers, respectively, to allow for sticky-end ligation. α2δ-3 was ligated to the linearized vector by blunt end ligation. The integrity of each probe was verified by dideoxy termination sequencing. 35S-UTP (DuPont NEN, Wilmington, DE)-labeled sense and antisense riboprobes were generated using a standard T3 and T7 polymerase in vitro transcription procedure (T3 and T7 from New England Biolabs, Beverly, MA). Unincorporated nucleotides were removed using Sephadex G50 columns (Pharmacia, Freiburg, Germany).
Messenger RNA in situ hybridization was performed on cryostat sections of mouse brain. A prehybridization step was performed at 45°C for 2 hr. Hybridization with 1 × 107cpm/ml probe proceeded for 16 hr at 55°C. After a 20 μg/ml RNase A (Boehringer Mannheim, Mannheim, Germany) digestion, a high-stringency wash of 0.1× SSC, 1 mm EDTA, and 1 mmdithiothreitol was done for 2 hr at 60°C. The slides were dehydrated in ethanol and analyzed by autoradiography.
Transfection of HEK293 cells and electrophysiological recordings. The full-length cDNAs of all subunits, i.e., α1C, α1E, β2a, β3, α2δa-1, and α2δ-3 were cloned into the pcDNA 3 vector (Invitrogen). For more details, see Schuster et al. (1996). HEK 293 cells were transfected with various combinations of an α1 subunit (α1C, α1E), a β subunit (β2a, β3), and an α2δ subunit (α2δ-1, α2δ-3). This was achieved by lipofection with Lipofectamine (Life Technologies) at a DNA mass ratio of 1:1 for expression of two subunits or 1:1:1 for three subunits.
Electrophysiological recordings. Ionic currents from transfected cells were recorded in whole-cell configuration of the patch-clamp method. Ba2+ was used as the charge carrier. The extracellular solution contained (in mm):N-methyl-d-glucamine, 125; BaCl2, 20; CsCl, 5; MgCl2, 1; HEPES, 10; and glucose, 5, pH 7.4 (HCl). The intracellular solution contained (in mm): CsCl, 60; CaCl2, 1; EGTA, 11; MgCl2, 1; K2ATP, 5; HEPES, 10; and aspartic acid, 50, pH 7.4 (CsOH). Currents were recorded using an EPC-9 patch-clamp amplifier and corresponding Pulse software from Heka Electronics (Lambrecht, Germany). Patch pipettes were pulled from borosilicate glass. The pipette input resistance was typically between 1.8 and 2.2 MΩ. The capacity of individual cells ranged between 25 and 90 pF, and series resistance ranged between 3.5 and 5.0 MΩ. Capacity transients were compensated using build-in procedure of the Heka system. Curve fitting and statistical analysis were performed using the Origin 5.0 software package (Microcal, Northampton, MA). The significance of observed differences was evaluated by nonpaired Student’s t test. Probability of 5% or less was considered to be significant.
RESULTS
Primary structures
A database search using the α2δ subunit cDNA as probe revealed three additional sequences similar to this calcium channel subunit (Fig. 1), which were derived from two genes named 2 and 3. Two of these sequences are human full-length sequences of closely related isoforms of the α2δ subunit (GenBank accession numbers: AF042792, isoform I; AF042793, isoform II; M. H. Wei, F. Latif, F. M. Duh, D. Andreazzoli-Angeloni, V. Kashuba, E. Zabarovsky, B. Johnson, and M. I. Lerman, unpublished observations). These sequences differ only at the N terminus of the α2 protein, indicating that both isoforms are derived from the same gene 2 and are generated by differential splicing. The 5′-untranslated region upstream of the ATG codon from isoform I is in good agreement with the Kozak sequence for initiation of translation in eukaryotic cells, whereas the same region of isoform II shows only a limited homology with this sequence. Furthermore, only isoform I but not isoform II shows features of a potential signal sequence. Both observations suggest that only isoform I can form a functional calcium channel subunit. Isoform I, which we describe here as the α2δ-2 subunit, (Wei, Latif, Duh, Andreazzoli-Angeloni, Kashuba, Zabarovsky, Johnson, and Lerman, unpublished observations) has 55.6% identity with the α2δ-1 subunit (Ellis et al., 1988). Eighteen potentialN-glycosylation sites can be identified in the primary structure (Fig. 1), which is the same number of sites as in the α2δ-1 subunit (Ellis et al., 1988).
Fig. 1.
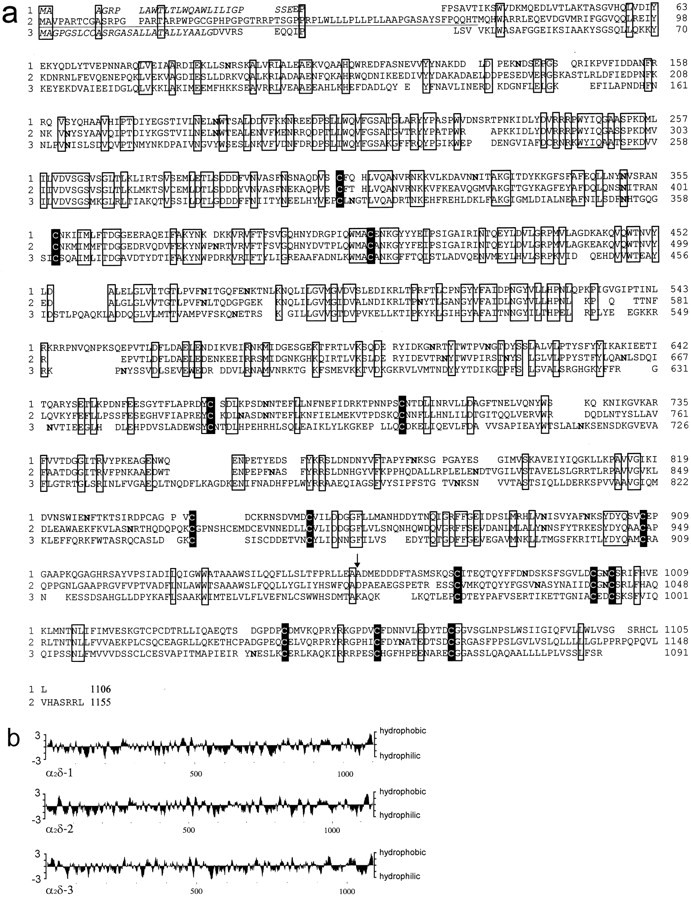
a, Amino acid alignment of the α2δ-1 (1), α2δ-2 (2), and α2δ-3 (3) subunits. The N-terminal region differing between the α2δ-2 subunit isoform I and II isunderlined. Regions that are identical in all sequences are boxed, and conserved cysteine residues are additionally highlighted. The presumptive signal peptides for classes 1 and 3 are shown in italics. Potential N-glycosylation sites are printed inbold. The arrow indicates the cleavage site between the α2 and δ proteins of the α2δ-1 subunit. These sequence data are available from the EMBL database under accession numbers M21948 for the α2δ-1 subunit, AF042792 for the α2δ-2(I) subunit, and AF042793 for the α2δ-2(II) subunit isoforms, respectively, and AJ010949for the α2δ-3 subunit. b, Hydrophobicity profile of the α2δ subunits computed according to Kyte and Doolittle (1982). The curve is the average of a residue-specific hydrophobicity index over a window of nine residues.
The third sequence identified by database searches was a partial human EST sequence AC: AA190607. This sequence was used to design primers for the amplification of cDNA from mouse tissues. The cDNA could be amplified only from mouse brain mRNA and not from other tissues (Fig.2). A cDNA library was constructed from mouse brain mRNA and screened with the PCR product as a probe. Several independent clones could be identified that encode the novel α2δ subunit, which we name α2δ-3 (Fig.1). A detailed restriction analysis of the different clones showed that there are no further isoforms in mouse brain. The sequence upstream of the start ATG is in agreement with that for the initiation of translation in eukaryotic cells (data not shown). The open reading frame consists of 3273 bp encoding a protein with 1091 amino acid residues. The α2δ-3 subunit has 30.3% identity with the α2δ-1 subunit and 31.2% with the α2δ-2 subunit. The primary structure of α2δ-3 contains nine potentialN-glycosylation sites (Fig. 1a).
Fig. 2.
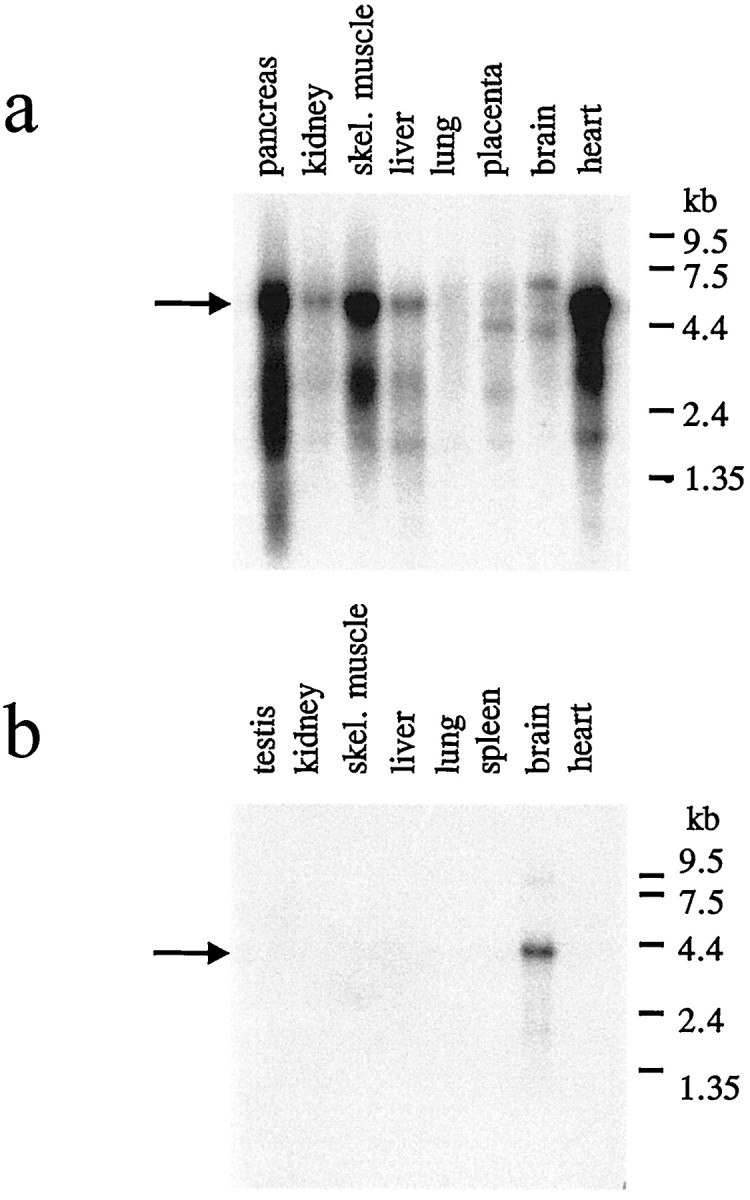
Northern blot analysis of the α2δ-2 and α2δ-3 subunits. For each lane, ∼2 μg of poly(A) RNA was run on a denaturing formaldehyde-containing agarose gel, transferred to a nylon membrane, and fixed by UV irradiation. a, Human multiple tissue blot using a specific probe for the α2δ-2 subunit.b, Mouse multiple tissue blot for the α2δ-3 subunit. Arrows indicate predominant species of mRNA with sizes of 5.2 (α2δ-2) and 4.3 (α2δ-3) kb.
Hydrophobicity analysis of all three α2δ subunits indicates a similar membrane topology, including a hydrophobic transmembrane segment at the C terminus of the δ domain (Fig.1b). A sequence comparison reveals that as many as 14 cysteine residues are conserved in all of the three α2δ genes, further strengthening the postulate that these subunits are disulfide-linked proteins with similar higher order structures.
Tissue distribution
The expression of the novel α2δ subunits was examined by Northern and in situ hybridization. The α2δ-2 subunit is highly expressed in heart, pancreas, and skeletal muscle tissue (Fig. 2), which can be seen even after 6 hr of autoradiography of Northern blots. After 1 d of exposure, the α2δ-2 subunit can also be detected in kidney, liver, placenta, and brain. This broad expression pattern was also observed for the α2δ-1 subunit. An 8.0 kb transcript of the α2δ-1 subunit was detected in brain, heart, aorta, skeletal muscle, and ileum (Ellis et al., 1988). In contrast to this ubiquitous expression pattern, the α2δ-3 subunit is only found in brain (Fig. 2). In each case, the Northern analysis reveals one predominant species of mRNA with sizes of 5.2 (class 2) and 4.3 kb (class 3).
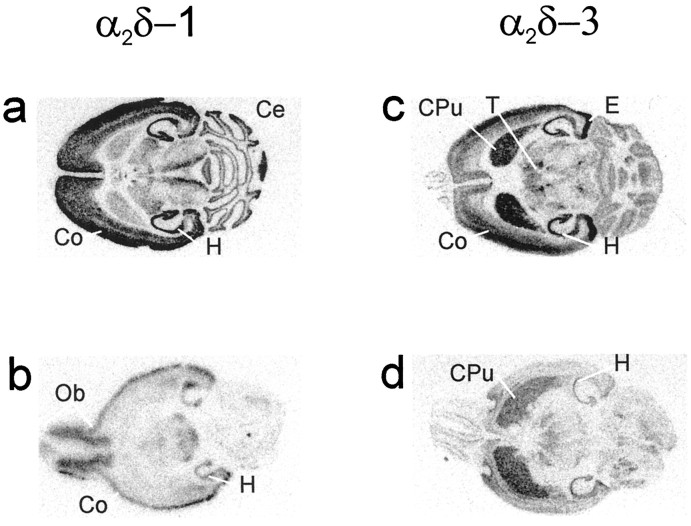
The mRNA expression of α2δ-1 and α2δ-3 calcium channel subunits in mouse brain was mapped by in situ hybridization. Both α2δ forms were detected in several regions of the brain, with differential expression of the mRNAs in some structures. Strong expression of α2δ-1 was seen in the pyramidal cell layer of Ammon’s horn in the hippocampus (CA1–3) and in the granular cell layer of the dentate gyrus (Fig. 3). The olfactory bulb also stained strongly, and expression in the mitral and glomerular cell regions was seen by dark-field microscopy of slides coated with photographic emulsion. Signals in the cerebellar cortex, and to a lesser extent in thalamic nuclei, were also observed (Fig. 3). Examination of emulsion-coated slides showed that the expression of α2δ-1 in the cerebellum was restricted to the granular layer (data not shown).
Fig. 3.
Autoradiographs of α2δ-1 and α2δ-3 riboprobe hybridization to horizontal mouse brain sections. Central (a, c) and more basal (b, d) sections of the brain are shown. Expression of α2δ-1 is seen in the (a) hippocampus (H), cerebral cortex (Co), cerebellum (Ce), and (b) olfactory bulb (Ob). α2δ-3 mRNA was detected in the caudate putamen (CPu), hippocampus (H), entorhinal complex (E), cortex (Co), and thalamic nuclei (T) (c, d).
The α2δ-3 mRNA was predominantly expressed in the caudate putamen, entorhinal complex, hippocampus, and cortex (Fig. 3). As with α2δ-1, the pyramidal cell layer of the hippocampus (CA1–3) and granular cell layer of the dentate gyrus showed the highest degree of hybridization with the antisense probe (Fig. 3). Hybridization of both probes was specific as judged by the absence of signals when sense probes were applied (data not shown).In situ hybridization for the α2δ-2 subunit indicated a ubiquitous expression in cardiac tissues (data not shown).
Functional characterization
Because the Northern blot hybridization indicated a high expression of the α2δ-3 subunit in brain, we performed cotransfection studies with α1 subunits that have been characterized in this tissue. Because in situ analysis did not show an exact colocalization of α2δ-3 with known α1 subunits, coexpression studies were done with an α1 subunit of the dihydropyridine-sensitive class C and a dihydropyridine-insensitive class E calcium channel. This approach allows for the identification of interactions with both evolutionary distant calcium channel α1 families.
Effects of the α2δ-3 subunit on barium current through the α1C-type calcium channel
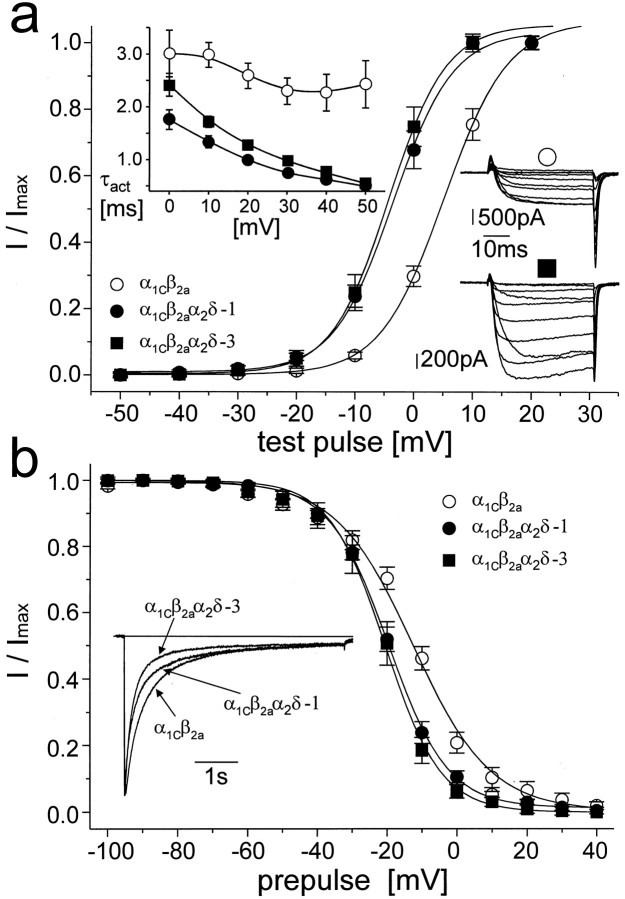
To study the effects of α2δ-3 subunit on channel gating, we coexpressed this subunit with the α1C subunit alone or in combination with both α1C and β2a subunits. Voltage protocols are described in detail in the legend to Figure4, and quantitative analyses are summarized in Table 1. When coexpressed with α1C alone, the effects of α2δ-3 on IBa were less noticeable than when β2a was coexpressed. α2δ-3 did not affect the current density, time course of current inactivation, and voltage dependence of steady-state inactivation of α1C expressed singly (data not shown). However, when expressed with α1C only, α2δ-3 shifted the activation curve by 4.4 mV in the hyperpolarizing direction and slightly accelerated the time of current activation during the depolarizing pulse (data not shown). The effects on the channel kinetics became more prominent in the presence of the β2a subunit (Fig. 4, Table 1). In this combination, α2δ-3 significantly increased the current density, shifted the voltage dependence of current activation by 8.7 mV in a hyperpolarizing direction, accelerated the time course of current activation during the depolarizing pulse, accelerated the current inactivation at positive membrane potentials, shifted the steady-state inactivation curve in a hyperpolarizing direction, and significantly changed its slope.
Fig. 4.
The α2δ subunit affects current through the α1C-type calcium channel. The α2δ-3 subunit was coexpressed with α1C and β2a. For comparison, the current through cells coexpressing α2δ-1 subunit was coanalyzed.a shows the voltage dependence of current activation measured as the amplitude of current activated by a 40-msec-long depolarizing pulse from a holding potential of −80 mV to voltages marked on the ordinate and normalized to the maximal amplitude. Each voltage dependence was fitted to the Boltzmann equation. Results of these fits are summarized in Table 1. The inset in thetop left of a shows voltage dependence of the kinetics of current activation. The ascending phase of the current time course was fitted to single exponential. The resulting time constants were averaged and plotted against corresponding membrane potentials. In both graphs, ○ represents the α1Cβ2a channel, • the α1Cβ2aα2δ-1 channel, and ▪ the α1Cβ2aα2δ-3 channel. Theinset in the right of aillustrates a typical family of currents measured during a series of depolarizing pulses from the holding potential of −80 mV to membrane potentials ranging from −20 to +70 mV with a step of +10 mV. ○, α1Cβ2a channel. The cell capacity was 83 pF, and the resulting maximal current density was approximately −13 pA/pF; ▪, α1Cβ2aα2δ-3. The cell capacity was 31 pF, and the corresponding maximal current density was approximately −41 pA/pF. b shows averaged steady-state inactivation curves measured from a holding potential of −80 mV. Current was inactivated by a 5-sec-long prepulse to the potentials marked on the ordinate. This was followed by a 5-msec-long return to the holding potential and a 40-msec-long test pulse to the maximum of the current–voltage relationship. Solid lines are fitted to the Boltzmann equation. The inset shows the time course of the current during a 5-sec-long depolarizing pulse to +20 mV with zero level indicated by a horizontal line. Currents shown are averaged time courses from 9 to 12 experiments scaled to the same amplitude. Individual measurements were fitted to the sum of two exponentials. Results of all fitting procedures are summarized in Table1. Symbols are as in a.
Table 1.
Comparison of the effects of α2δ-1 and α2δ-3 subunits on IBa through α1C or α1E-type calcium channels
| α1Cβ2a | α1Cβ2aα2δ-1 | α1Cβ2aα2δ-3 | α1Eβ3 | α1Eβ3α2δ-1 | α1Eβ3α2δ-3 | |
|---|---|---|---|---|---|---|
| IBa density (pA/pF) | 13.8 ± 1.4 (16) | 30.3 ± 6.6* (15) | 28.6 ± 4.9* (21) | 24.4 ± 5.4 (12) | 98.1 ± 20.0*** (10) | 95.4 ± 15.6*** (10) |
| V0.5 activ. (mV) | +5.2 ± 0.1 (16) | −5.0 ± 0.6*** (13) | −3.5 ± 0.5*** (11) | −13.0 ± 1.1 (10) | −14.5 ± 0.2 (9) | −20.1 ± 0.7***, aaa (11) |
| kactiv | 5.4 ± 0.1 | 5.1 ± 0.4 | 5.1 ± 0.4 | 5.6 ± 0.9 | 3.7 ± 0.1 | 4.6 ± 0.6 |
| V0.5 inact. (mV) | −12.5 ± 0.9 (9) | −19.6 ± .3*** (14) | −20.5 ± 0.4*** (8) | −64.4 ± 0.4 (9) | −69.7 ± 0.4*** (11) | −76.2 ± 0.5***, aaa (9) |
| kinact | 11.1 ± 0.7 | 8.5 ± 0.2*** | 8.0 ± 0.3** | 7.1 ± 0.4 | 8.4 ± 0.4* | 9.5 ± 0.4*** |
| A1 (%) | 61.1 ± 3.9 (12) | 73.7 ± 4.5* (11) | 78.1 ± 2.6** (9) | 93.0 ± 2.1 (8) | 97.3 ± 0.5* (10) | 98.5 ± 0.5** (10) |
| τ1 (msec) | 273 ± 24 | 260 ± 19 | 156 ± 10***, aaa | 31.9 ± 5.7 | 23.6 ± 0.9 | 24.1 ± 1.7 |
| τ2 (sec) | 1.16 ± 0.08 | 3.54 ± 0.46*** | 0.90 ± 0.10aaa |
The data are the means ± SEM. The number of experiments performed is indicated in parentheses. The first row compares the average current density for each channel. The second row compares voltage dependence of current activation of different subunit combinations. The ascending part of the I–Vcurve, which is shown in panel a of Figures 4 and 5 was fitted to the Boltzmann function. V0.5 activ and kactiv are the parameters of these fits. The fourth row compares voltage dependence of current inactivation. The steady-state inactivation curves shown in panel b of Figures 4 and 5 were fitted to the Boltzmann function. V0.5 inact and kinact are parameters of Boltzmann fits. The last three rows show the results of fitting the time course of inactivation ofIBa during a 5-sec-long (α1C-derived channels, traces shown in Figure4b) or 300-msec-long (α1E-derived channels, traces shown in Figure 5b) depolarizing pulse to +20 mV. Currents through α1C-derived channels were fitted to a sum of two exponentials. A1 represents the relative amplitude of the current inactivating with the fast time constant τ1. α1E-derived channels were found to inactivate with single time constant. For these channels, 100-A1 represents the relative amplitude of the noninactivating component of the current remaining at the end of the depolarizing pulse (see also Fig. 5b). The significance of the difference was tested using the unpaired Student’st test. Asterisks (*) indicate the statistical significance between α1Cβ2a and α1Cβ2aα2δ-1 or α1Cβ2aα2δ-3 and between α1Eβ3 and α1Eβ3α2δ-1 or α1Eβ3α2δ-3. Significant difference between α1Cβ2aα2δ-1 and α1Cβ2aα2δ-3 and between α1Eβ3α2δ-1 and α1Eβ3α2δ-3 is marked by (a).
*p < 0.05; **p < 0.01; ***p < 0.001; aaap < 0.001.
We further compared the effects of α2δ-3 with those of the previously described α2δ-1 subunit (Fig. 4, Table1). The changes in gating-related channel characteristics elicited by both subunits were qualitatively similar, and in most cases the measurements (current density, voltage dependence of current activation and inactivation, and time course of current activation) were not significantly different. Both α2δ-1 and -3 subunits accelerated the time course of current inactivation at a membrane potential of +20 mV by enhancing the proportion of the fast time constant, τ1. α2δ-3 changed this constant from 273 ± 24 msec to 156 ± 10 msec. Surprisingly, α2δ-1 significantly increased the value of the slow time constant τ2 from 1.16 ± 0.08 sec to 3.54 ± 0.46 sec, but this effect was apparently overwhelmed by the effect of the increased proportion of the current inactivating with fast time constant τ1, with the result that the overall time course of inactivation was accelerated (Fig. 4b, inset). The effects of both α2δ-3 and α2δ-1 subunits on whole-cell current parameters, which reflect the gating of the α1C channel, are virtually identical and they require the presence of β (in this case β2a) to become prominent.
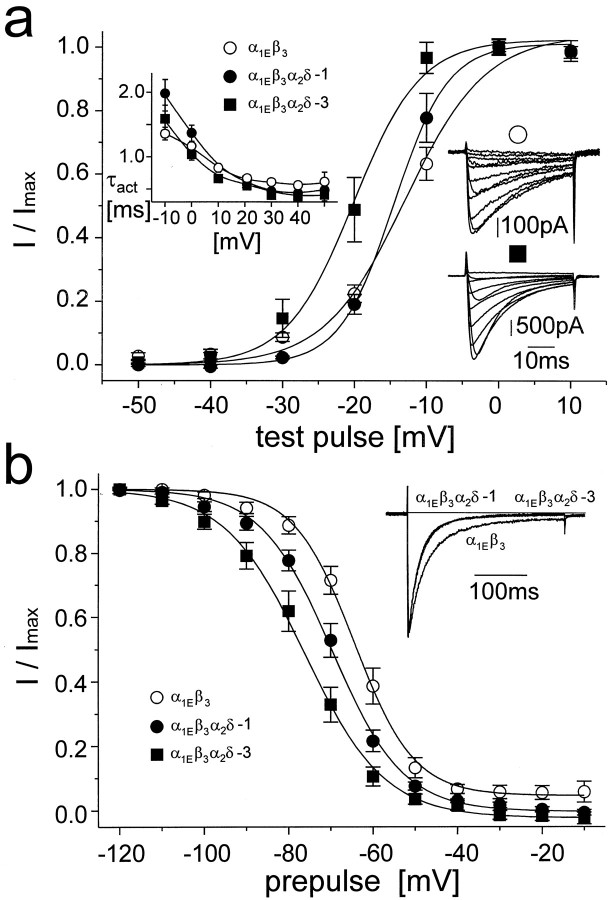
Effects of the α2δ-3 subunit on barium current through α1E-type calcium channel
Although both α1C and α2δ-1 subunits are fairly abundant in mammalian tissues, α1E and α2δ-3 are predominantly expressed in neuronal tissue. We therefore selected α1E for studying the effects of α2δ-3. For all experiments, β3 subunit was coexpressed with α1E. This β subunit was suggested to modulate the current through the α1E channel (Ludwig et al., 1997). As with the α1C channel, both α2δ-1 and α2δ-3 affected most of the gating-related parameters except for the time constant of current activation during membrane depolarization (Fig.5, Table 1). In contrast to α1Cβ2a channel, the effects of α2δ-3 and α2δ-1 on the voltage dependence of current activation (Fig. 5a) and inactivation (Fig. 5b) of α1Eβ3 channel were significantly different. The α2δ-1 subunit shifted both activation and steady-state inactivation curves in a hyperpolarizing direction, but the change in current activation was not statistically significant. In both curves, the shift evoked by α2δ-3 was significantly larger (Table 1). In contrast, the effects of both α2δs on the time course of current inactivation at a membrane potential of +20 mV were identical and restricted to diminution of the noninactivating part of the current (Table 1).
Fig. 5.
The α2δ subunit affects current through the α1E-type calcium channel. Unless otherwise indicated, the voltage protocols used were the same as those described in the legend to Figure 4. ○ represents the α1Eβ3 channel; • the α1Eβ3α2δ-1 channel, and ▪ the α1Eβ3α2δ-3 channel. Boltzmann fits of voltage dependencies of current activation are summarized in Table 1. The inset in theright of a shows a typical family of currents measured during a series of depolarizing pulses from a holding potential of −100 mV to membrane potentials ranging from −30 mV to +60 mV with step of +10 mV. ○, α1Eβ3 channel, with a cell capacity of 19 pF and a maximal current density of approximately −34 pA/pF; ▪, α1Eβ3α2δ-3 channel, with a capacity of 43 pF and a maximal current density of approximately −74 pA/pF. b shows the steady-state inactivation curve measured from a holding potential of −100 mV using a 5-sec- long conditioning pulse to membrane potentials marked on the ordinate.Solid lines represent Boltzmann fits. Theinsert illustrates the inactivation ofIBa during a 300-msec-long depolarizing pulse from a holding potential of −100 mV to +20 mV scaled to the same amplitude. Eight to 10 measurements were averaged for each channel type. The α1Eβ3α2δ-1 and α1Eβ3α2δ-3 current traces are indistinguishable from each other. Individual time courses of current inactivation were fitted to a single exponential with a small proportion of noninactivating current. Results of all fits are summarized in Table 1. Symbols are as ina.
DISCUSSION
We present here the first account of the existence of multiple α2δ calcium channel subunits. The previously known α2δ we have named α2δ-1, and the new forms have been designated α2δ-2 and α2δ-3 based on their similarity to the original subunit. An amino acid alignment reveals that there is only a significant degree of homology in the core region of the α2 protein, whereas the δ proteins show identities only with respect to the cysteine residues. Despite the low degree of homology at the primary structure level between these forms, other features such as the number of glycosylation sites, cysteine residues, and hydrophobicity profiles are very similar. For these reasons we conclude that all three α2δ subunits are disulfide-linked proteins with similar higher order structures. The α2δ-1 and -2 subunits are more ubiquitously expressed than α2δ-3, which has only been identified in brain. Comparisons of the expression patterns of the 1 and 3 class α2δ subunits with α1 subunits in brain slices (Ludwig et al., 1997) do not indicate specific α1-α2δ combinations. We cannot exclude the possibility that α2δ-3 also interacts with other ion channels or even other membrane proteins.
Functional coexpression of the α2δ-1 subunit with various combinations of α1 and β subunits results in an increase in the current densities or dihydropyridine (DHP)-binding sites (Singer et al., 1991; Welling et al., 1993;De Waard et al., 1995; Shistik et al., 1995; Bangalore et al., 1996;Gurnett et al., 1996; Felix et al., 1997; Parent et al., 1997;Jones et al., 1998), acceleration of current activation and inactivation (Singer et al., 1991; De Waard et al., 1995; Bangalore et al., 1996; Felix et al., 1997; Qin et al., 1998; Shirokov et al., 1998), and a shift of the current–voltage curve in a hyperpolarizing direction (Singer et al., 1991; Felix et al., 1997). Regardless of the α1 subunit used in this study, the α2δ-3 subunit was found to increase the current density, which is in agreement with the results of these groups. In addition to this effect, coexpression of α2δ-3 caused a shift of the voltage dependence of channel activation and inactivation in a hyperpolarizing direction and an acceleration in the kinetics of current inactivation.
When the results shown in Figures 4 and 5 are compared, it can be seen that α2δ-1 caused a smaller shift in the voltage dependence of activation and inactivation of α1Eβ3 as compared with the α1Cβ2a channel. The effects of α2δ-3 on the electrophysiological properties of α1C coexpressed with β2a was found to be similar to those of α2δ-1. In contrast, coexpression of α2δ-3 with α1E and β3 produced more pronounced differences in the current characteristics associated with channel gating than the coexpression of α2δ-1.
The mechanism whereby α2δ modulates the conductances of α1 is not clearly understood. The increase in density of current and DHP-binding sites can be explained by an improved targeting of expressed α1 subunit to the cell membrane and maturation of the channel complex (Shistik et al., 1995), which leads to an increased amount of charge moved during channel activation (Bangalore et al., 1996; Qin et al., 1998). It was suggested that the increase in current requires the presence of an intact α2protein, whereas the shift of voltage-dependent activation and steady state inactivation as well as the acceleration of the inactivation kinetics are caused by the transmembrane δ protein (Felix et al., 1997). However, based on the amino acid similarity of the three subunit forms, it seems more likely that α2 harbors the relevant residues responsible for the observed effects on α1 and that the δ domain functions only as an membrane anchor for α2. This interpretation is further strengthened by the sequences of the δ proteins, which are not conserved.
These results, together with the brain-specific expression of α2δ-3, suggest that the α2δ-3 subunit may have a distinct physiological role in neuronal tissue. The α2δ protein has been implicated as the in vivo target for the antiepileptic drug gabapentin (Gee et al., 1996), which apparently inhibits calcium currents in isolated rat brain neurons (Stefani et al., 1998). It was proposed that gabapentin binds preferentially to the α2δ-1 subunit. This is supported by evidence that the partial N-terminal amino acid sequence of the gabapentin-binding protein obtained from porcine brain membranes is identical with an aminoterminal peptide of α2δ-1. This sequence is not present in classes 2 or 3. Further support for this postulate is that our mRNA in situ analysis of α2δ-1 in brain showed the same distribution as that of gabapentin-binding sites (Taylor et al., 1998), which differs from that of α2δ-3. For these reasons it is more likely that gabapentin targets the α2δ-1 subunit. However, another study using a different purification procedure showed that there may be an additional gabapentin-binding protein in brain, which was detected with a polyclonal α2 antibody (Brown et al., 1998) and which could be another α2δ form. Further investigations need to be undertaken to elucidate the binding of gabapentin to the α2δ subunit.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft and Fond der Chemie.
Correspondence should be addressed to Dr. Norbert Klugbauer, Institut für Pharmakologie und Toxikologie der Technischen Universität München, Biedersteiner Strasse 29, 80802 München, Germany.
Dr. Lacinová contributed to this paper while on leave from Institute of Molecular Physiology and Genetics, Slovak Academy of Sciences, Vlarska 5, 833 04 Bratislava, Slovakia.
REFERENCES
- 1.Angelotti T, Hofmann F. Tissue-specific expression of splice variants of the mouse voltage-gated calcium channel α2δ subunit. FEBS Lett. 1996;397:331–337. doi: 10.1016/s0014-5793(96)01205-7. [DOI] [PubMed] [Google Scholar]
- 2.Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel α2/δ-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am J Physiol. 1996;270:H1521–H1528. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- 3.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel α1-subunit in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 4.Brickley K, Campbell V, Berrow N, Leach R, Norman RI, Wray D, Dolphin AC, Baldwin SA. Use of site-directed antibodies to probe the topography of the α2 subunit of voltage-gated Ca2+ channels. FEBS Lett. 1995;364:129–133. doi: 10.1016/0014-5793(95)00371-f. [DOI] [PubMed] [Google Scholar]
- 5.Brown JP, Dissanayke VUK, Briggs AR, Milic MR, Gee N. Isolation of the [3H] gabapentin-binding protein/α2/δ Ca2+ channel subunit from porcine brain: development of a radioligand binding assay for α2/δ subunits using [3H]leucine. Anal Biochem. 1998;255:236–243. doi: 10.1006/abio.1997.2447. [DOI] [PubMed] [Google Scholar]
- 6.Cribbs LL, Lee J-H, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- 7.De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- 8.De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. J Physiol (Lond) 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberst R, Dai S, Klugbauer S, Hofmann F. Identification and functional characterization of a calcium channel gamma subunit. Pflügers Arch. 1997;433:633–637. doi: 10.1007/s004240050324. [DOI] [PubMed] [Google Scholar]
- 10.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 11.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. J Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee NS, Brown JP, Dissanayake VUK, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 13.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 15.Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 16.Jones LP, Wei S-K, Yue DT. Mechanisms of auxiliary subunit modulation of neuronal α1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 18.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parent L, Schneider T, Moore CP, Talwar D. Subunit regulation of the human brain α1E calcium channel. J Membr Biol. 1997;160:127–140. doi: 10.1007/s002329900302. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J-H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–899. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 22.Qin N, Olcese R, Stefani E, Birnbaumer L. Modulation of human neuronal α1E-type calcium channel by α2δ-subunit. Am J Physiol. 1998;274:C1324–C1331. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- 23.Schuster A, Lacinová L, Klugbauer N, Ito H, Birnbaumer L, Hofmann F. The IV26 segment of the L-type calcium channel is critical for the action of dihydropyridines and phenylalkylamines. EMBO J. 1996;15:2365–2370. [PMC free article] [PubMed] [Google Scholar]
- 24.Shirokov R, Ferreira G, Yi J, Ríos E. Inactivation of gating currents of L-type calcium channels. Specific role of the α2δ subunit. J Gen Physiol. 1998;111:807–823. doi: 10.1085/jgp.111.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and α1 protein level. J Physiol (Lond) 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 27.Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 28.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 29.Taylor CP, Gee NS, Su T-Z, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 30.Welling A, Bosse E, Cavalié A, Bottlender G, Ludwig A, Nastainczyk W, Flockerzi V, Hofmann F. Stable co-expression of calcium channel, α1, β and α2/δ subunits in a somatic cell line. J Physiol (Lond) 1993;471:749–765. doi: 10.1113/jphysiol.1993.sp019926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiser O, Trus M, Tobi D, Halevi S, Giladi E, Atlas D. The α2/δ subunit of voltage sensitive Ca2+ channels is a single transmembrane extracellular protein which is involved in regulated secretion. FEBS Lett. 1996;379:15–20. doi: 10.1016/0014-5793(95)01475-6. [DOI] [PubMed] [Google Scholar]