Fig. 8.
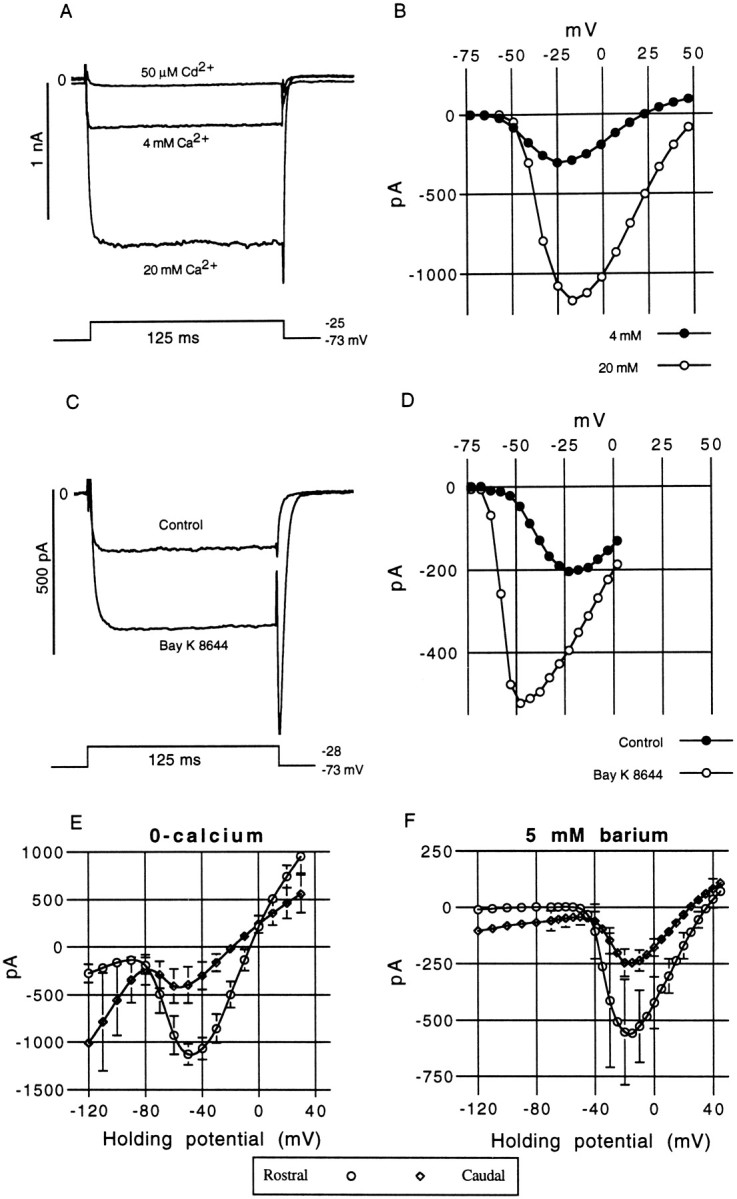
Ionic sensitivity and pharmacology of the inward calcium current. A, The inward current revealed by a CsCl internal pipette solution could be modulated by changes in [Ca+2]ext. The control solution used in most experiments contained 4 mm calcium. Increasing the external concentration to 20 mm caused an approximately fourfold increase in the size of the inward current. In contrast, the addition of 50 μm cadmium to the control solution caused a rapid but reversible block of the inward current.B, I–V curve derived for the same cell in 4 and 20 mm[Ca+2]ext, from a holding potential of −73 mV. Oscillatory medial hair cell, 11 pF; 9 MΩ; 80% src. C, Bay K 8644 (5 μm), an L-type calcium channel agonist, caused an increase in the steady-state and peak tail-current amplitudes, which is attributed to an extension in the single channel mean open time. The tail current becomes substantially slower with Bay K 8644. This drug not only increases the steady-state amplitude over the entire voltage range (D) but produces a much steeper activation slope (the peak inward current is shifted from −23 to −48 mV) and appears to hyperpolarize the threshold of activation. Medial hair cell, 10 pF; 16 MΩ; 80% series resistance compensation (src). E, The mean steady-state I–V curves for rostral (n = 10) and caudal (n = 10) hair cells recorded in zero external calcium. Error bars are shown where they exceed the size of the symbols. The steady-state reversal potentials were −8 (rostral) and −19 mV (caudal). F, The mean steady-state I–Vcurves for rostral (n = 12) and caudal (n = 9) hair cells recorded in 5 mmexternal barium. The steady-state reversal potentials were +36 (rostral) and +25 mV (caudal).
