Abstract
Extracellular Zn2+ modulates current passage through voltage- and neurotransmitter-gated ion channels, at concentrations less than, or near, those produced by release at certain synapses. Electrophysiological effects of cytoplasmic Zn2+ are less well understood, and effects have been observed at concentrations that are orders of magnitude greater than those found in resting and stimulated neurons. To examine whether and how neurons are affected by lower levels of cytoplasmic Zn2+, we tested the effect of Zn2+-selective chelators, Zn2+-preferring ionophores, and exogenous Zn2+ on neuronal somata during whole-cell patch-clamp recordings. We report here that cytoplasmic zinc facilitates the downward regulation of a background Cl− conductance by an endogenous protein kinase C (PKC) in fish retinal ganglion cell somata and that this regulation is maintained if nanomolar levels of free Zn2+ are available. This regulation has not been described previously in any tissue, as other Cl− currents have been described as reduced by PKC alone, reduced by Zn2+ alone, or reduced by both independently. Moreover, control of cation currents by a zinc-dependent PKC has not been reported previously. The regulation we have observed thus provides the first electrophysiological measurements consistent with biochemical measurements of zinc-dependent PKC activity in other systems. These results suggest that contributions of background Cl− conductances to electrical properties of neurons are susceptible to modulation.
Keywords: background chloride conductance, resting potential, outward rectification, PKC, Zn2+, divalent cation
Calcium, magnesium, and zinc are found in both bound and ionized forms in neuronal and muscle cytoplasm. Of these, the concentration and physiological effects of unbound intracellular calcium (Ca2+) have been studied most extensively. Fluorescent indicators and patch-clamp methods have shown substantial agreement between the endogenous free Ca2+ levels measured during certain electrophysiological responses and buffered levels of exogenous Ca2+ that can elicit those events (e.g., Johnson and Byerly, 1993; Roberts, 1993; Burgoyne and Morgan, 1995; Etter et al., 1996). Recent measurements suggest a similar correlation for Mg2+, in that free intracellular Mg2+ concentrations range from 0.6 to 5 mm (Brocard et al., 1993) and thus span the range of Mg2+ levels that gate or regulate ion channels (e.g., Matsuda et al., 1987; Stelzer et al., 1988; Johnson and Ascher, 1990; O’Rourke et al., 1992). By comparison, electrophysiological effects of cytoplasmic Zn2+ have been reported rarely, and these effects have been obtained with Zn2+ concentrations that are several orders of magnitude greater than the picomolar-to-micromolar levels found in recent histochemical studies (Begenisich and Lynch, 1974; Woll et al., 1987; Frederickson, 1989; Kokubun et al., 1991; Groschner and Kukovetz, 1992; Staley, 1994; Lascola et al., 1998; Sensi et al., 1997). We therefore tested whether and how neurons are affected electrophysiologically by lower concentrations of cytoplasmic Zn2+ and by changes in these levels. For this purpose, we applied various combinations of Zn2+, Zn2+-selective chelators, and Zn2+-preferring ionophores to isolated neuronal somata during perforated- and ruptured-patch whole-cell patch-clamp recordings. We specifically tested for these effects in neurons that contain protein kinase C (PKC) (cf. Cuenca et al., 1990; Osborne et al., 1992), because biochemical studies have shown that Zn2+ binds PKC and facilitates its activity at submicromolar concentrations (Murakami et al., 1987; Csermely et al., 1988; Sekiguchi et al., 1988; Forbes et al., 1991; Hubbard et al., 1991). We present evidence here that intracellular zinc facilitates the reduction of a “background” Cl− conductance (Franciolini and Petris, 1990) by an endogenous PKC in retinal ganglion cells.
The results below are presented in two parts. The first identifies an outwardly rectifying Cl− current that constitutes a background Cl− conductance in retinal ganglion cells. The second part provides evidence that regulation of this current by endogenous PKC is zinc dependent.
Portions of these data have appeared in a meeting abstract (Tabata and Ishida, 1997).
MATERIALS AND METHODS
Whole-cell recordings. The voltage-clamp currents described here were measured in single, neurite-free retinal ganglion cell somata isolated from adult common goldfish (Carassius auratus). Cells were isolated and identified as described elsewhere (see Bindokas et al., 1994; Tabata and Ishida, 1996; Hidaka and Ishida, 1998). Currents were measured in tight-seal whole-cell configurations, in either ruptured- or perforated-patch mode (Hamill et al., 1981; Horn and Marty, 1988). Experiments were performed at ∼23°C within 20 hr of cell isolation, using borosilicate glass pipettes with tip resistances of 2–3 MΩ and an Axopatch-1D amplifier (Axon Instruments, Foster City, CA).
During recording, cells were continuously superfused with a control bath solution (see below) at a rate of 1.2 ml/min. To reduce the time required to change extracellular solution composition and to ensure that current changes were not attributable to mechanical artifacts, we applied control and test solutions sequentially to cells, either by switching between fluid reservoirs fed into a microperfusion U-tube or by shifting the position of a parallel array of glass tubes that each superfused a different solution over the cells being recorded. As described elsewhere (Tabata and Ishida, 1996), command potential generation, data storage, and off-line analysis were performed with pCLAMP software (version 6.0.3; Axon Instruments); capacitive currents were reduced as much as possible by use of the cancellation circuitry of the amplifier; current signals were analog-filtered at 1 kHz and digitally sampled at 2 kHz; and liquid junction potentials between pipette and bath solutions were measured and corrected for before data collection. Membrane potentials are reported without compensation for series resistance (mean ± SEM; 18 ± 1 MΩ in ruptured-patch mode; n = 65; 32 ± 4 MΩ in perforated-patch mode; n = 46) because the total membrane current rarely exceeded 200 pA. Unless otherwise indicated, data are reported here as mean values ± 1 SEM from the indicated number (n) of cells. The current amplitudes reported are the mean values recorded during the final 20 msec of 100 msec steps to each test potential. Changes in current amplitudes measured under various conditions were compared by Wilcoxon rank-sum tests because these values did not distribute normally. Variances of data measured with different pipette solutions (e.g., see Fig. 3C) were compared by F tests (by comparing the ratio of the variances).
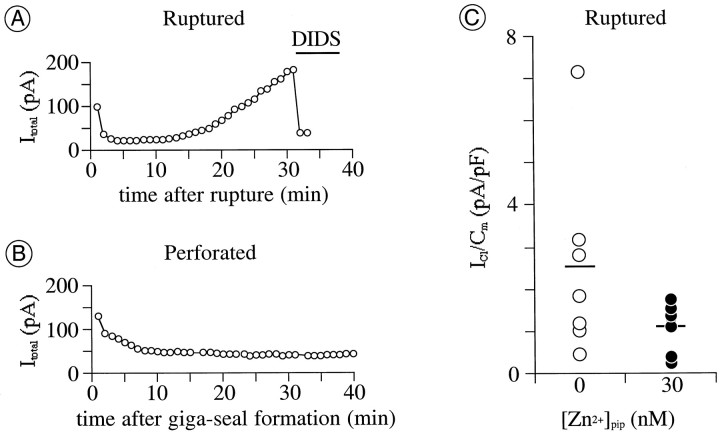
Fig. 3.
Exogenously supplemented intracellular Zn2+ impedes augmentation ofICl by intracellular perfusion.A, B, Amplitude of total current atEtest of +40 mV plotted against recording time. Recordings in A and B were in ruptured- and perforated-patch mode, respectively. Pipette solutions in both recordings contained no added zinc ([Zn2+]pip = 0); 1 mmDIDS was microperfused onto the cell during the time indicated by thehorizontal bar in A.Cm, 10 pF in A and 28 pF in B. C,ICl activated by depolarization to +40 mV after 12–15 min of intracellular perfusion with Zn2+-free versus Zn2+-containing pipette solutions. Each circle plots the increase in amplitude of ICl over this time for a different cell, normalized by the Cm of that cell. Horizontal bars plot the mean of values for each pipette solution [mean ± SD; 2.6 ± 2.3;n = 7 cells for [Zn2+]pip = 0 nm(open circles); 1.1 ± 0.6; n = 6 for [Zn2+]pip = 30 nm (filled circles)].
Solutions. The compositions of the routinely used pipette and bath solutions are as follows (exceptions are noted in the figure legends). In ruptured-patch mode, the 30 nmZn2+-containing pipette solution consisted of (in mm): 150 N-methyl-d-glucamine (NMDG), 0.226 CaCl2, 1.7 MgCl2, 3.6 ZnCl2, 2 ATP-Mg, 4 EGTA, and 5 HEPES. The Cl− concentration in this solution (11 mm) was used for the comparison of equilibrium and reversal potentials (see Fig. 2). The Zn2+-free pipette solution contained (in mm): 150 NMDG, 2.48 CaCl2, 1.73 MgCl2, 2 ATP-Mg, 4 EGTA, and 5 HEPES. The Zn2+-free and Zn2+-containing pipette solutions contained different amounts of total Ca2+ but identical amounts of calculated free Ca2+ (see below). The pH of both of these solutions was adjusted to 7.5 withd-gluconic acid (DGA). In some experiments, the PKC catalytic subunit (#539513; Calbiochem, La Jolla, CA), and PKC[19–31] (#1443 976; Boehringer Mannheim, Indianapolis, IN) were first dissolved into water to a concentration 1000 times higher than the final one and then diluted into the Zn2+-free pipette solution to the final concentration immediately before experiments. In perforated-patch mode, the pipette tip was filled with a solution containing (in mm): 150 CsOH, 3.5 CaCl2, 4 MgCl2, 10 BAPTA, and 5 HEPES; pH was adjusted to 7.5 with DGA. The pipette shank was filled with the above solution mixed at 500:1 with a solution of 3.3% (w/v) amphotericine B (Sigma, St. Louis, MO) and 10% (w/v) pluronic (P-1572; Molecular Probes, Eugene, OR) in DMSO.
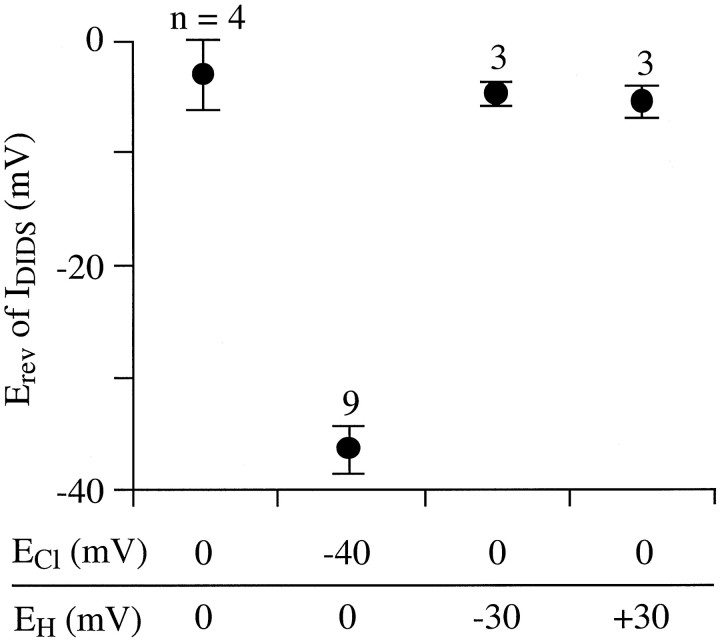
Fig. 2.
Cl−, not H+, carries DIDS-sensitive current. Interpolated values of reversal potential (Erev) of DIDS-sensitive current (measured as described in Fig.1B) listed next to Cl− and H+ equilibrium potential values (ECl andEH, respectively) set by bath and pipette solution compositions. Ruptured-patch recording mode was used. The control bath solution contained 53 mmCl−, the bath Cl− was reduced to 11 mm by isosmotic replacement with DGA. The pipette solution contained 11 mm Cl− and 30 nm free Zn2+ (see Materials and Methods for other constituents). Filled circles and error bars plot the mean ± 1 SEM of Erev measured from the indicated number of cells. Erev of DIDS-sensitive current shifts with ECl but not with EH.
The concentrations of free divalent cations in each pipette solution (reported in the text and each figure legend) were calculated using the equations in Chang et al. (1988) and the stability constants in Smith and Martell (1975) corrected for ionic equivalent, pH, and temperature according to the method of Marks and Maxfield (1991). EGTA was used instead of BAPTA in these experiments, because dissociation constants for the binding of Zn2+ by BAPTA are not available. In the cases of Zn2+-free solutions, the free Ca2+ and Mg2+ values calculated as described above did not differ by >20% from those calculated using the Bound and Determined software (Brooks and Storey, 1992) implementing the method of Marks and Maxfield (1991). Unless stated otherwise, the free Ca2+ concentration in all pipette solutions was set to levels detected in resting retinal ganglion cells by fura-2 fluorescence intensity [100 nm(Bindokas et al., 1994)]. The free Zn2+concentration in the pipette solution routinely used for ruptured-patch recordings (30 nm) was selected because it falls within the range of concentrations measured in a variety of intact cells (cf.Sensi et al., 1997) and because micromolar Zn2+ has consistently been found to inhibit PKC activity in biochemical studies (Murakami et al., 1987; Csermely et al., 1988; Sekiguchi et al., 1988). We know of no method that could have been used during the ruptured-patch recordings reported here to demonstrate the precise distribution and concentration of Zn2+ established in the cell cytoplasm by exchange with the pipette solution.
The standard bath solution contained (in mm): 105 Na-DGA, 18 NaCl, 0.001 tetrodotoxin (TTX), 0.1 CaCl2, 2.4 CoCl2, 30 tetraethylammonium (TEA)-Cl, 3 4-aminopyridine (4-AP), 10 d-glucose, and 5 HEPES; pH was adjusted to 7.5 with NaOH and/or HCl. To prepare a test bath solution, we first dissolved a test agent into an appropriate vehicle solvent to a concentration 1000 times higher than the concentration to be applied. This stock solution was kept at −30°C for up to 1 week and diluted into the bath solution to the final concentration immediately before experiments. The following solvents were used: water for Rp-cAMP (Calbiochem) and pyrithione-Na (Aldrich, Milwaukee, WI); ethanol forN,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN; Calbiochem); methanol for 4,4′-di-isothiocyanostilbene-2,2′-disulfonic acid (DIDS; Aldrich) and 4-acetamido-4′-isothiocyanostilbene-2–2′-disulfonic acid (SITS; Aldrich); and DMSO for bisindolylmaleimide I (Calbiochem), calphostin C (Calbiochem), and 4-bromo-A23187 (4-BrA23187; Calbiochem). The control bath solution contained the same vehicle solvent as the corresponding test bath solution.
Three precautions were exercised routinely during this study. First, the ruptured-patch pipette solution, the perforated-patch pipette solution, and bath solutions were adjusted with sucrose to 320 ± 5, 330 ± 5, and 330 ± 5 mOsm/kg, respectively. With these osmolalities, neither swelling nor shrinkage of cells occurred after giga-seal formation. Second, DIDS-sensitive current was measured in individual cells by digital subtraction of currents recorded before and during a single application of DIDS, because multiple applications irreversibly altered the kinetics and degree ofICl deactivation in many cells and because retinal ganglion cells possess a K+ current (IB) that resists block by TEA, 4-AP, and Cs+ (Lukasiewicz and Werblin, 1988; Sucher and Lipton, 1992). Changes of ICl amplitude produced by pharmacological treatments that augment or block PKC activity were gauged either with a single DIDS application at the end of an experiment or by comparison of the reversal potential and kinetics of the difference between currents recorded before and during the pharmacological treatments. Third, voltage-gated cation currents were suppressed as follows: Na+ current was blocked by inclusion of 1 μm TTX, Ca2+ currents were blocked by 2.4 mm Co2+ and reduced Ca2+ (0.1 mm), K+currents (except IB) were blocked by 30 mm TEA and 3 mm 4-AP, and hyperpolarization-activated cation current (Ih) was suppressed by excluding K+ from the bath solution (see Bindokas et al., 1994; Tabata and Ishida, 1996; Hidaka and Ishida, 1998).
RESULTS
In the presence of pharmacological blockers of voltage-gated Na+, K+, and Ca2+ currents (TTX, TEA+, 4-AP, and Co2+), depolarization of retinal ganglion cell somata activated an outwardly rectifying current whose amplitude was reduced by agents that block voltage-gated Cl−current in other tissues. This current appeared to be carried by Cl− ions—and will be referred to hereafter asICl—because its reversal potential shifted with the chloride equilibrium potential (ECl) calculated from the Cl− ion concentrations in the bath and pipette solutions used. A current with similar voltage sensitivity and pharmacological properties was also recorded when Na+ and K+ in the bath and recording pipette solutions were replaced by NMDG and TEA+. ICl activated in every cell from which we recorded, in ruptured- as well as perforated-patch recording modes (n = 65 and 51, respectively). When activated by 100 msec depolarizations from holding potentials between −90 and −70 mV, to test potentials between −80 and +40 mV,ICl displayed the following pharmacological properties and voltage sensitivity.
Pharmacology
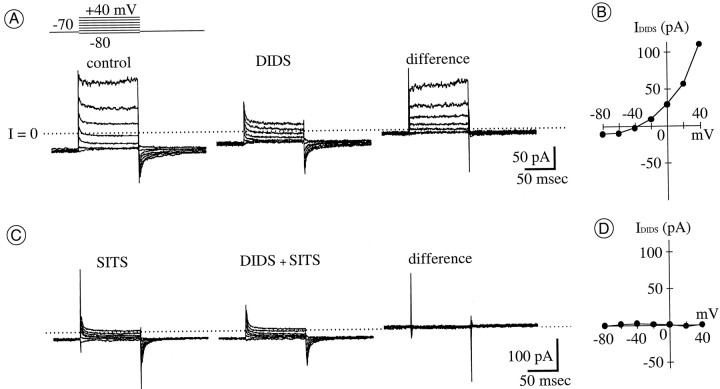
Several Cl− channel blockers were tested, and total whole-cell current (between −80 and +40 mV) was reduced in amplitude by extracellular application of DIDS (n = 61; e.g., Fig. 1A) and furosemide (data not shown). This reduction by DIDS and the outward rectification recorded under control conditions (Fig.1B) were not observed if SITS was included in the pipette solution in ruptured-patch mode (consistent with block of the DIDS-sensitive current by intracellular SITS; n = 6; Fig. 1C,D). By contrast, whole-cell current was unaffected by extracellular applications of 100 μmpicrotoxinin or 500 nm chlorotoxin (n = 3 and 6, respectively; data not shown).
Fig. 1.
DIDS and SITS reduce an outwardly rectifying current in the presence of Na+, Ca2+, and K+ current blockers. A, Whole-cell current activated in ruptured-patch mode by the voltage protocol schematically shown above current traces atleft. The holding potential (Ehold) was −70 mV; test potentials (Etest) were from −80 to +40 mV, in 20 mV increments. Currents were recorded before (left) and during (middle) application of 1 mmDIDS. Subtraction of these currents yields DIDS-sensitive current (right). The difference current appears to activate rapidly, not inactivate, and then deactivate rapidly (at onset, plateau, and offset, respectively, of each test depolarization). In this and all other current traces, the zero current level is shown by a dotted line. B, Current–voltage (I–V) curve of the DIDS-sensitive current in A. In this and all otherI–V curves, current amplitude is averaged over the final 20 msec of 100 msec steps to each test potential and then plotted against test potential. Current reverses direction nearECl (−40 mV). C, Whole-cell current recorded from a cell different from that in Abefore (left) and during (middle) application of 1 mm DIDS. Current was activated and recorded as described in A, except that 100 μm SITS was included in the recording pipette solution. Ionic current recorded under this condition is unaffected by 1 mm DIDS; the difference between currents before and during DIDS (right) is due to slight capacitive current changes only. D, I–V curve measured from the difference of currents in C. The calculated free concentration of Zn2+ in the pipette solution ([Zn2+]pip) was 30 nm.
Nearly maximal reduction of the control current amplitude was produced by 1 mm DIDS. The current that resisted block by 1 mm DIDS constituted approximately one-third of the total outward current recorded at a test potential of +40 mV. When normalized to cell capacitance (Cm), the amounts of current that resisted block by DIDS resembled the total whole-cell current recorded (at +40 mV) with pipette solutions that contained 300 μmSITS (0.4 ± 0.4 pA/pF; n = 6; see Fig.1C). These DIDS- and SITS-resistant currents were not examined in detail. However, after repolarization to the holding potential, the amplitude of the “tail” of these currents was K+-sensitive (n = 31; data not shown). We presume that this current is analogous to the TEA- and 4-AP–resistant cation current termed IBin retinal ganglion cells of other species (Lukasiewicz and Werblin, 1988; Sucher and Lipton, 1992) and that it is carried by other cations (primarily Na+ in ruptured-patch mode and Cs+ in perforated-patch mode) under our recording conditions, as in other neurons (Zhu and Ikeda, 1993; Callahan and Korn, 1994). This observation indicates that, at the concentrations used, DIDS did not abolish voltage-sensitive currents nonselectively in the cells from which we recorded.
Charge carrier and Ca2+ insensitivity
Two results indicated that 1 mm DIDS reduced the amplitude of a Cl− current in retinal ganglion cells under the recording conditions used here. First, as mentioned above, this current reversed in direction at a membrane potential that shifted with extracellular Cl− concentration (Fig.2). When the external Cl− concentration was changed from 53 to 11 mm (by isosmotic replacement with d-gluconic acid, with the internal Cl− concentration fixed at 11 mm), the reversal potential of the DIDS-sensitive current shifted from −36 ± 2 mV (n = 9) to −3 ± 3 mV (n = 4). These results suggest that this current is carried at least primarily by Cl−ions, as characteristically found in background Cl−currents of various cells (Franciolini and Petris, 1990). (From the bath and pipette solution compositions, the equilibrium potentials for Na+ and Ca2+ ions are estimated to have been extremely positive values and to have remained constant while the Cl− reversal potential changed during these measurements.) Second, the reversal potential of the DIDS-sensitive current was unaffected by a 59 mV shift in the H+ equilibrium potential (produced by 0.5 pH unit increases and decreases in extracellular pH; n = 6; Fig. 2). Our records thus showed no detectable amounts of the DIDS-sensitive proton current described in human macrophages (Holevinsky et al., 1994).
Two results indicated that the DIDS-sensitive current was not gated by intracellular Ca2+. First, no increases in current amplitude were detected when cells were exposed to a divalent cation ionophore (4-BrA23187) in the presence of 0.1 mmCa2+ (n = 5; see Fig. 5 and its description below). Second, the DIDS-sensitive current density (current amplitude normalized to cell membrane capacitance) did not significantly differ when measured in ruptured-patch mode with pipette solutions containing calculated free Ca2+ levels of 10, 30, and 300 nm (n = 4, 5, and 3, respectively; data not shown). Although the density ofICl was indistinguishable when these different pipette solutions were used, the total depolarization-activated outward current density more than doubled at +40 mV (the test potential used routinely to characterize ICl) when these solutions contained K+ rather than NMDG as the major monovalent cation and when the calculated free Ca2+level was increased from 10 to 300 nm. These results are consistent with the presence of a Ca2+-activated K+ conductance in goldfish retinal ganglion cells, not unlike that suggested by measurements in salamander, turtle, rat, and cat retinal ganglion cells (see Ishida, 1995). By contrast, the activation of the ICl we report here apparently does not require increases in cytoplasmic Ca2+ and thus differs from the Ca2+-activated Cl− current of rod and cone photoreceptors, retinal bipolar cells, and other central neurons (Bader et al., 1982; Maricq and Korenbrot, 1988; Okada et al., 1995).
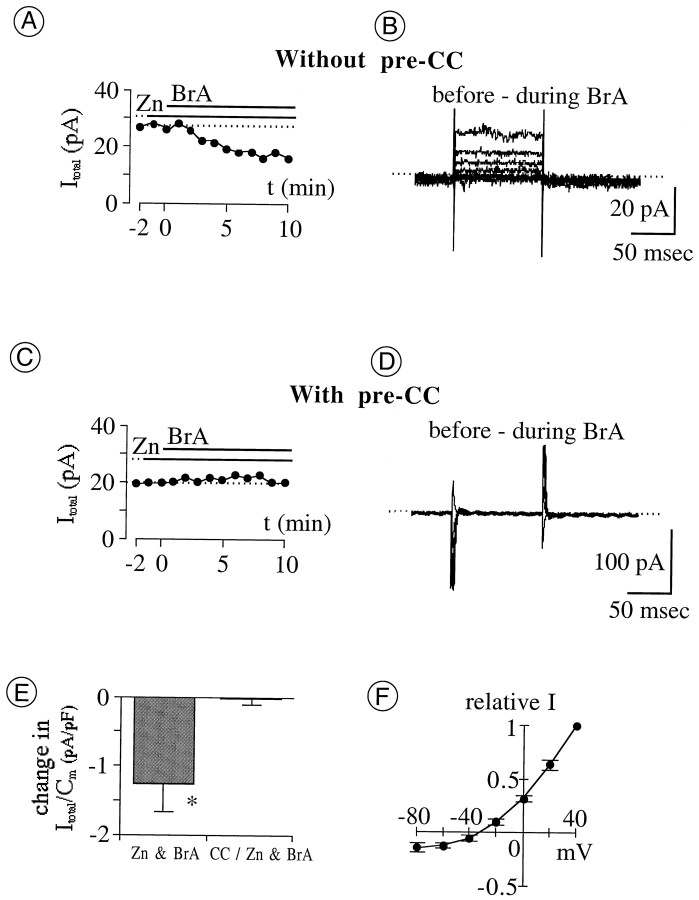
Fig. 5.
Effects of Zn2+ “loading” and PKC inhibition on total whole-cell current (Itotal) in perforated-patch mode. The cytoplasmic Zn2+ level was raised by extracellular application (indicated by horizontal bars) of ZnCl2 (Zn) together with the Zn2+-preferring ionophore 4-BrA23187 (BrA). Endogenous PKC was blocked by preincubation with calphostin C (CC). A, C, Amplitude of Itotal at +40 mV plotted against recording time. Each panel is from a single cell. The dotted line indicates current level before coapplication of Zn and BrA.A, Sequential application of 100 μmZn and 10 μmBrA.Itotal begins to decline after coapplication commences (at t = 0 min); within the next 8 min, current reduction is maximal. C, Sequential application of 10 μmZn and 10 μmBrA, preceded by a 30 min preincubation in 1 μmCC. Itotalremains constant during the 10 min coapplication of Znand BrA. B, D, Difference between Itotal recorded 1 min before and 10 min after coapplication of Zn and BrA. Current traces in B and Dare from cells in A and C, respectively. The voltage protocol is as described in Figure 1(Ehold, −70 mV;Etest, −80 to +40 mV; 20 mV steps).E, Reduction in Itotal after 10 min coapplication of Zn and BrA, with and without 30 min preincubation in 1 μmCC. Filled vertical bars and error bars plot the mean and SEM, respectively, of the reduction in current amplitude at +40 mV, divided by Cm to normalize for cell size. Current reduction by Zn is significantly greater without CC than withCC (*p = 0.0058), indicating that reduction of ICl by Zn can be blocked by inhibition of endogenous PKC. CC/Zn & BrA, Coapplication of 10 μmZn and 10 μmBrA after preincubation (n = 5); Zn & BrA, coapplication of 1–10 μmZn and 10 μmBrA without preincubation (n = 7).F, I–V curve of current reduced by coapplication of Zn (1–10 μm) andBrA (10 μm). Filled circlesplot the mean of difference current amplitudes measured as described inB (n = 6). Means are normalized to the value at +40 mV. Error bars indicate 1 SEM.Erev, −34.5 ± 5 mV.
On the basis of the above results and to obviate the need for leak-current subtraction, we measured ICl in the remainder of this study by the difference between whole-cell current recorded before and during application of 1 mm DIDS. The amplitude, reversal potential, and kinetics ofICl were measured from these “difference currents.” In some cases, we also inferred thatICl was susceptible to regulation by pharmacological agents that affect protein kinase activities, if the difference currents recorded before and during application of these agents resembled the DIDS difference currents.
Gating in ruptured- and perforated-patch modes
The activation range of ICl included membrane potentials that were more negative and more positive than theECl routinely used in our experiments (−40 or −32 mV); ICl was inward at test potentials more negative than ECl, and it was outward at more positive test potentials (see Figs. 1B,7H). The chord conductance measured from outward currents exceeded that measured from inward currents when the bath and pipette solutions contained equal Cl−concentrations and when the standard bath and pipette solutions were used (bath, 53 mm Cl−; pipette, 11 mm Cl−). When the bath and pipette solutions both contained 11 mm Cl−, for example, the outward ICl at +40 mV was 2.9 ± 0.3 (n = 4) times larger in amplitude than the inward ICl measured at −40 mV.
Fig. 7.
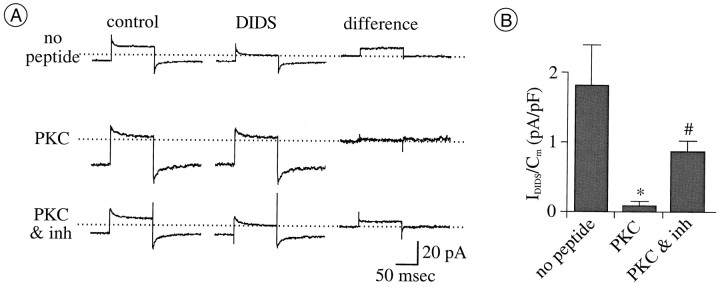
Downregulation ofICl by PKC-mediated phosphorylation.A, Currents activated by depolarization from −70 to +40 mV before (left column) and during (middle column) application of 1 mm DIDS and with digital subtractions thereof (right column). Eachrow of currents is from a separate cell, with the ruptured-patch pipette solution containing no peptide (top row), 4 pm PKC catalytic subunit (middle row), or a mixture of 4 pm PKC catalytic subunit and PKC inhibitor [1 μm PKC(19–31)] (bottom row). DIDS was applied to each cell 9–15 min after formation of ruptured-patch–recording mode. The flat difference current in the middle row shows that there is no current blocked by DIDS in the presence of PKC. The DIDS-sensitive difference current in the bottom row showsICl spared by PKC inhibition.B, Density of DIDS-sensitive currents measured as described in A. Filled vertical bars and error bars plot the mean ± SEM; n = 5 for each treatment. In each recording, the pipette solution contained 2 mm ATP and no added Zn2+. Mean density is significantly reduced by PKC from the control (no peptide) level (*p = 0.0122). Mean density with PKC(19–31) is significantly larger than those with PKC only (#p = 0.0122).
Over the range of test potentials we routinely used (i.e., between −80 and +40 mV), ICl did not exhibit markedly time-dependent gating kinetics. First, its rise time was both rapid and not obviously voltage dependent; the rising edge of currents measured after depolarizations to test potentials between −40 and +40 mV could all be fitted by single-exponential time constants of 3–5 msec. Second, ICl was not detectably inactivated by depolarization because it did not decline in amplitude during sustained depolarizations (Fig. 1) and because the currents activated by depolarizations from −90 to +20 mV were identical in amplitude to those activated by depolarizations from −30 to +20 mV (n = 5; data not shown). Third, tail currents were immeasurably small, and thus ICl displayed no time-dependent deactivation (Fig. 1).
It was possible to activate ICl of stable amplitude repeatedly in perforated-patch mode but not in ruptured-patch mode with the standard pipette solution. This was gauged, as explained above, in two steps. The total whole-cell current was measured during the last 20 msec of 100 msec depolarizations, repeated once per 60 sec, from a holding potential of −70 mV to a test potential of +40 mV; 1 mm DIDS was then applied to determine the fraction of the total current that was comprised by ICl. During ruptured-patch recordings, the amplitude of the total test current increased continuously and by as much as 10-fold or more during the course of 30 min (Fig. 3A). We infer that this increase in total whole-cell current is attributable primarily, if not entirely, to increases in IClbecause 1 mm DIDS blocked approximately all of the total current that (by the end of each recording) exceeded the levels recorded at the beginning of each recording (Fig. 3A; the possibility that higher concentrations of DIDS might produce larger reductions in the whole-cell current was not tested). During these long-term recordings, the slowly rising current recorded during the first few milliseconds of each test depolarization and the total whole-cell current tail recorded after repolarization to the holding potential did not markedly change in amplitude. These current components therefore vanished upon subtraction of the current traces recorded in the presence and absence of DIDS (Fig. 1), leaving traces that show no time-dependent activation or deactivation (ofICl). The steady growth ofICl illustrated by Figure 3A was never seen in perforated-patch mode. Instead, the amplitude of the total current decreased within the first 5–10 min of giga-seal formation and was stable thereafter for at least 30 min (Fig.3B). The initial decline in current amplitude presumably reflects the slow replacement of intracellular K+ by Cs+ in the pipette solution.
It was technically infeasible to quantify the rate at whichICl increased over time in ruptured-patch mode by alternating applications of control and DIDS-containing bath solution, because applications of DIDS produced changes in the deactivation kinetics of the total current. Nevertheless, the above results imply that at least some of the current measured during 100 msec test depolarizations may be regulated via an intracellular molecule or mechanism that was lost or compromised by exposure to the ruptured-patch pipette solution. These results also indicate that the slowly rising and tail current components can both be activated repeatedly, without substantial change, in ruptured- as well as perforated-patch recordings.
Regulation of ICl by a zinc-dependent mechanism
We tested the possibility that the increase in amplitude ofICl during recordings in ruptured-patch mode resulted from chelation of cytoplasmic Zn2+ by EGTA in the pipette solution rather than from chelation of Ca2+. We tested this possibility because Zn2+ has been detected in the cytoplasm of various central neurons (Frederickson, 1989) and because EGTA binds Zn2+ with a greater affinity than it does other divalent cations (e.g., Ca2+ and Mg2+; see Materials and Methods). A first test of this possibility was made by including Zn2+ in the pipette solution used in ruptured-patch mode. When Zn2+ was included in the pipette solution at a free concentration calculated to be 30 nm, the density ofICl (measured at 12–15 min after membrane rupture) ranged from 0.3 to 1.8 pA/pF (mean ± SD; 1.1 ± 0.6; n = 6). Without an exogenous supplement of Zn2+, ICl density ranged from 0.5 to 7.2 pA/pF (mean ± SD; 2.6 ± 2.3; n = 7; Fig. 3C). The variance of the current densities measured with the Zn2+-free pipette solution was significantly larger than that measured with the Zn2+-containing pipette solution (p < 0.01, F test), suggesting that the growth of ICl was often facilitated by the replacement of cytoplasmic constituents by a Zn2+-free solution and that this growth was hindered by the inclusion of Zn2+ in the pipette solution. Large increases in ICl were not observed with pipette solutions containing a calculated free Zn2+concentration of 3 nm (n = 2). However,ICl densities were not compared in detail at different pipette Zn2+ concentrations because we had no means to ascertain the Zn2+ levels established within cells during the recordings attempted here. Under both conditions studied (30 nm vs no free Zn2+), the difference between currents measured before and after DIDS application displayed outward rectification, rapid activation, no inactivation, and no time-dependent deactivation.
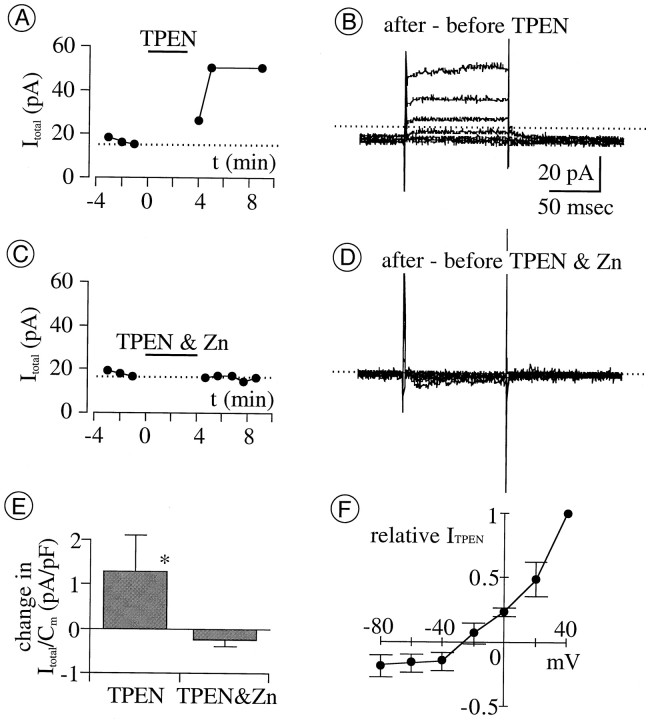
A second test of whether the availability of cytoplasmic Zn2+ abated the growth of IClwas made by superfusing cells with the membrane-permeable Zn2+-selective chelator TPEN (Arslan et al., 1985) in perforated-patch mode. At 10 μm, TPEN increased the amplitude of the total whole-cell current at a test potential of +40 mV (Fig.4A,E). The TPEN-augmented difference current (obtained by subtraction of currents recorded before and after application of TPEN) was indistinguishable from ICl in that it rectified outwardly, reversed direction at −27 ± 3 mV (whenECl was −32 mV; n = 5; see Fig.4 for details), and lacked the inward tail component recorded both in the presence and absence of DIDS (Fig. 1A). This effect of TPEN was prevented by coapplication (of TPEN) with Zn2+ (Fig. 4C,D), as expected because TPEN is membrane impermeant when it has complexed with Zn2+ (Arslan et al., 1985).
Fig. 4.
The membrane-permeable Zn chelator TPEN augments total whole-cell current (Itotal).A, Amplitude of Itotalactivated by depolarization from −70 to +40 mV, in perforated-patch mode, before and after application of TPEN (10 μm; indicated by horizontal bar) is shown. Here and in all subsequent figures, application of the test agent of interest begins at 0 min; current measurements before 0 min (with or without conditioning agents) are plotted against negative time values. Note that the increase in current peaks at 6–8 min after TPEN application begins.B, Difference between currents activated before and after TPEN at test potentials between −80 and +40 mV is shown.C, D, TPEN (10 μm) fails to augment Itotal when coapplied with equimolar Zn2+. E, The current density change produced by TPEN and by a mixture of TPEN and Zn2+at +40 mV is shown. Current density measured 8 min after drug application began, minus that at 1 min before drug application, is plotted (filled vertical bars and error bars plot mean ± 1 SEM, respectively; n = 5 for each treatment). The mean change with TPEN is significantly larger than that with TPEN and Zn2+ (*p = 0.0216). F, I–V relation of TPEN-augmented current is shown (filled circlesand error bars plot mean ± SEM, respectively;n = 5). Amplitude is normalized to the value at +40 mV and plotted against test potential.Erev, −27 ± 3 mV;ECl, −32 mV. In all panels, currents were recorded in the control bath solution before TPEN application. TPEN was then superfused for 3–4 min after replacing the bath Co2+ with Ca2+ (because TPEN binds Co2+ more than Ca2+ and only unbound TPEN can pass through cell membranes). Effects of TPEN were assessed by recording currents after replacing the TPEN- and Ca2+-containing solution with control (Co2+-containing) solution to bind residual TPEN (if any) and to block voltage-gated Ca2+ currents.
A third test of the effect of cytoplasmic Zn2+ onICl was made by microperfusing a mixture of Zn2+ plus an ionophore that conducts Zn2+ onto cells during recordings in perforated-patch mode. As shown in Figure5A, the amplitude of outward current activated at a test potential of +40 mV declined in amplitude (Fig. 5A,B,E) after the coapplication of 1–100 μm Zn2+ either with 4-BrA23187 [10 μm (Erdahl et al., 1996)] or with pyrithione [20–100 μm (Zalewski et al., 1993)]. When coapplied with 4-BrA23187, 1 μm Zn2+(n = 3), 10 μm Zn2+(n = 5), and 100 μmZn2+ (n = 3) produced declines of similar amplitude. Neither Zn2+ nor 4-BrA23187 alone reduced Itotal at the concentrations used here, and moreover, similar declines in Itotal were produced by the coapplication of Zn2+ and 4-BrA23187 when the coapplication was preceded by an application of Zn2+ alone (n = 8) or of 4-BrA23187 alone (n = 3). This suggests that the decline inItotal resulted from rises in intracellular Zn2+ concentration and not from a 4-BrA23187–mediated influx of Ca2+ or from an effect of Zn2+ on the external membrane surface. The zinc-damped difference current obtained by subtraction of currents recorded before and after these treatments was indistinguishable fromICl in that it rectified outwardly, reversed direction close to ECl (Fig.5F), and displayed no time-dependent deactivation. The decline produced by coapplication of Zn2+ and pyrithione was small (0.5 pA/pF; n = 2) and not examined in detail.
Downward regulation of ICl by an endogenous, zinc-dependent PKC
The results described in the preceding section suggest that the amplitude of whole-cell ICl is downwardly regulated, either directly or indirectly, by cytoplasmic Zn2+. We tested the possibility that this effect is exerted indirectly for four reasons: (1) because studies of direct Zn2+ effects on Cl− channels in other cell types used substantially higher Zn2+concentrations than those used here (see introductory remarks), (2) because biochemical assays have shown that (aside from modulating ion channel activities) Zn2+ facilitates PKC activity (e.g., Murakami et al., 1987; Csermely et al., 1988), (3) because PKC has been found to modulate Cl− currents in a variety of cells (e.g., Madison et al., 1986; Li et al., 1989; Kokubun et al., 1991; Tricarico et al., 1991; Kawasaki et al., 1994; Staley, 1994; Coca-Prados et al., 1995; Duan et al., 1997), and (4) because PKC has been localized by immunocytochemical methods to retinal ganglion cells of the species used here (Osborne et al., 1992). We therefore tested whether ICl is regulated by endogenous PKC activity and whether this control was zinc dependent.
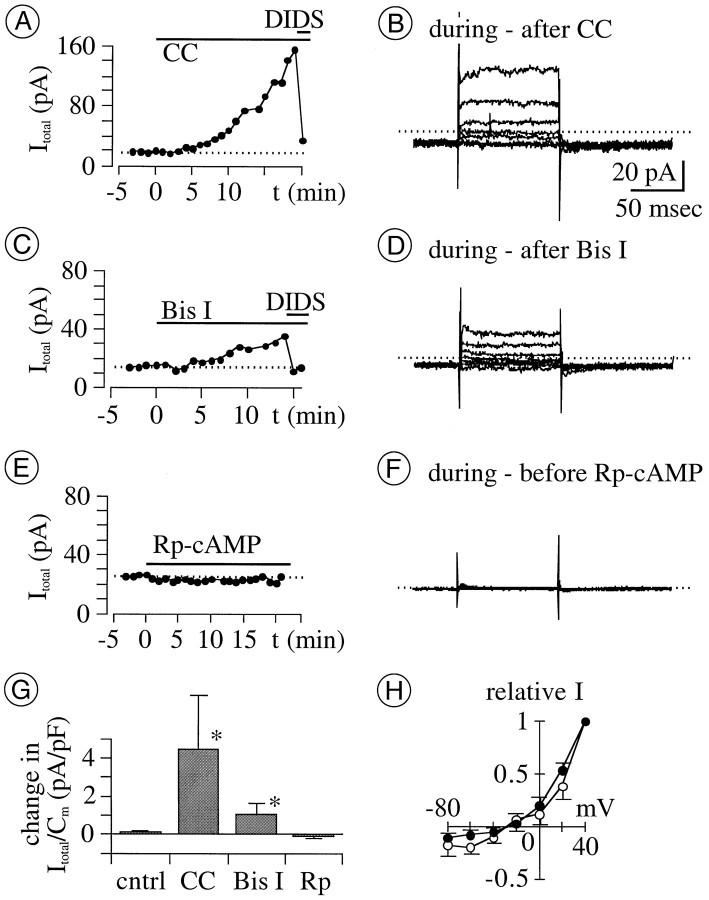
We first tested the effect of extracellularly applied PKC inhibitors onICl in perforated-patch mode. Both agents tested (0.1–1 μm calphostin C and 1 μmbisindolylmaleimide I) (cf. Shapiro et al., 1996) produced an increase in the amplitude of the total outward current (n = 4 for each agent; Fig. 6G). At a test potential of +40 mV, current amplitude increased noticeably (e.g., by 10–20%) over the control value within 4–8 min after application of either agent (Fig.6A,C). Current amplitude continued to increase thereafter during applications of either agent for as long as 10–15 min. We infer that increases in the amplitude ofICl account for most (if not all) of the increases in outward current observed, because the latter were reduced by DIDS (1 mm) and because the kinetics and voltage sensitivity of the difference current traces obtained by subtraction of currents recorded before and after exposure to PKC inhibitors (Fig.6B,D,H) resemble those of the Cl− current described above (Fig. 1). These results suggest that ICl is reduced in resting retinal ganglion cells by an endogenous PKC activity.
Fig. 6.
Effect of membrane-permeable PK inhibitors onICl in perforated-patch mode.A, C, E, Amplitude of total current (Itotal) at +40 mV before and after application (indicated by horizontal bars) of inhibitors of PKC and PKA. Each recording is from a different cell. Concentrations used were 0.1 μmcalphostin C (CC), 1 mm DIDS, 1 μm bisindolylmaleimide I (Bis I), and 0.1 mm Rp-cAMP. Amplitudes ofItotal start to increase 4–8 min afterCC (A) and Bis I(C) applications begin, and grow continuously and gradually thereafter. DIDS (applied at times indicated by short horizontal bars) reduces current toward the levels beforeCC and Bis I. B,D, F, Difference between currents recorded before and after 13–21 min application of various test agents. Data in B, D, andF were obtained from the same cells shown inA, C, and E, respectively. Calibration (in B) is the same in allpanels. The kinetics of difference currents inB and D (i.e., of currents augmented byCC and by Bis I) resembles those of ICl in Figure 1. G, Mean change in density of Itotal at +40 mV after 15–20 min application of various test agents. Bis I, 1 μmBis I; CC, 0.1–1 μmCC; cntrl, control bath solutions supplemented with vehicle (DMSO) only; Rp, 0.1–1 mm Rp-cAMP (n = 4 for each treatment). The mean change with CC and Bis I is significantly larger than the control level (*p > 0.0209 for both). H,I–V relation of current augmented by CC(filled circles) and Bis I(open circles). Amplitude was normalized to the maximum value recorded from each cell at +40 mV and plotted against test potential. Symbols and error bars plot the mean ± SEM, respectively (n = 4 for each treatment). Voltage sensitivity of these currents resembles those ofICl in Figure 1.Erev of CC- and Bis I-augmented currents, −27 ± 8 and −28 ± 3 mV, respectively; ECl, −32 mV.
As might therefore be expected, DIDS-sensitive current was undetectably small when PKC catalytic subunit (∼4 pm) was included in pipette solutions during ruptured-patch recordings (n = 5; Fig. 7A). Moreover, DIDS-sensitive current (ICl) could be activated when a PKC inhibitor (PKC[19–31]) (cf. Lin et al., 1994) and PKC catalytic subunit were included together in the pipette solution during ruptured-patch recordings (n = 5 for each treatment; Fig. 7). Therefore, the observed reduction ofICl amplitude cannot be ascribed to nonspecific effects of the PKC catalytic subunit (i.e., effects beside phosphorylation).
Lastly, we tested whether the downward regulation ofICl by endogenous PKC and that by cytoplasmic Zn2+ were linked. This was done by measuringItotal in the presence of calphostin C, Zn2+, and 4-BrA23187. Figure 5, C andE, shows that 10 μm Zn2+and 10 μm 4-BrA23187 did not reduce the amplitude ofItotal in cells incubated for 30 min in 1 μm calphostin C. The subtraction of currents recorded in the presence of all three agents from those recorded in the presence of Zn2+ and calphostin C alone showed no measurable difference current at any of the test potentials used (n = 5; Fig. 5D; only slight changes in capacitive current artifacts are seen at the onset and offset of the test potentials). The current reduction by Zn2+ was significantly less after ≥30 min preincubations with calphostin C than without it (Fig. 5E; p = 0.0058), indicating that inhibition of endogenous PKC impeded the reduction ofICl by Zn2+. Consistent with this and with the lag observed between calphostin C application andICl augmentation (e.g., Fig. 6),ICl amplitude grew monotonically if 10 μm Zn2+ and 10 μm4-BrA23187 were coapplied after a 10 min (rather than 30 min) exposure to 1 μm calphostin C (n = 5; data not shown). All of these results would be expected if the reduction ofICl by cytoplasmic Zn2+ (like that shown in Fig. 5A) was mediated by PKC.
Under the same recording conditions in which PKC inhibitors increasedICl, the Rp-isomer of cAMP (0.1–1 mm) produced no detectable change inICl when applied onto cells by microperfusion for as long as 25 min (Fig. 6G,H). Because Rp-cAMP is a membrane-permeable inhibitor of protein kinase A (PKA) and because the concentrations used are 10–100 times greater than its Ki, we did not further test whether endogenous PKA activity regulatesICl.
DISCUSSION
We have described here an outwardly rectifying Cl− current (ICl) whose amplitude appears to be downwardly regulated in a zinc-dependent manner by an endogenous PKC. Below, we discuss the role of zinc in suppressing this current, compare this current with anionic conductances in other cells, and delimit speculation about the function of this current.
Indirect effects of Zn2+
Previous studies showed that the open time of outward single-channel Cl− currents is reduced by application of 1–10 mm Zn2+ to the cytoplasmic side of isolated membrane patches and interpreted these effects as resulting from a voltage-dependent block (Woll et al., 1987;Kokubun et al., 1991; Groschner and Kukovetz, 1992). Our results might seem similar in that exposure of cells to Zn2+ and either 4-BrA23187 or pyrithione reduced the amplitude of outward whole-cell Cl− current (Fig. 5). However, our results differ from these previous reports in at least three respects. First, both inward and outward Cl− current amplitudes were augmented by pharmacological treatments that chelate cytoplasmic Zn2+ (exposure to TPEN; buffering divalent cation levels in nominally Zn2+-free ruptured-patch pipette solutions with EGTA); conversely, both currents were reduced by treatments designed to augment intracellular Zn2+ levels (Figs. 3A,4B, 5B). These results are not readily explained by assuming a voltage-dependent block by Zn2+ like that considered in the outward Cl− current measurements cited above. Second, inclusion of 30 nm free Zn2+ in our pipette solutions was effective in minimizing Cl−current increases in the ruptured-patch recording mode. This implies that ICl may be controlled by Zn2+ concentrations several orders of magnitude lower than those applied in the studies cited above (without excluding the possibility that higher Zn2+ concentrations might hinder Cl− channel gating in retinal ganglion cells) (cf. Staley, 1994). Third, we found that the effect of exogenously supplied cytoplasmic Zn2+ was blocked by the PKC inhibitor calphostin C. This suggests thatICl was not inhibited by Zn2+alone. If increased cytoplasmic Zn2+ levels reducedICl independently of PKC in our recordings, then these decreases were so small that they were outweighed by the increases in ICl produced by PKC inhibition (Fig. 5E).
The similarly slow and marked growth in Cl− current amplitude we observed after exposure to calphostin C and bisindolylmaleimide I, superfusion with TPEN, and internal perfusion with EGTA (without added Zn2+) would all be expected if ICl was downregulated by a PKC whose activity depends on Zn2+. Alone, any one of these results would have corroborated the results of previous studies, as Cl− current blockade by Zn2+ at the cytoplasmic side of membranes and the downward regulation of Cl− current by PKC have been described separately in other preparations (cited both above and below). Furthermore, PKC has been localized immunocytochemically to retinal ganglion cells in all species examined to date (e.g., Cuenca et al., 1990; Usuda et al., 1991; Osborne et al., 1992; Kolb et al., 1993; Fukuda et al., 1994). To our knowledge, however, the zinc dependence of the downward regulation of a Cl− current by PKC that we report here is novel. For that matter, our study provides the first electrophysiological evidence consistent with biochemical measurements of zinc-dependent PKC activity in other systems (Murakami et al., 1987;Csermely et al., 1988; Hubbard et al., 1991). This possibility was not predictable from immunocytochemical studies, as several PKC isozymes have been localized to retinal ganglion cells (e.g., Usuda et al., 1991; Osborne et al., 1992; Kolb et al., 1993), Zn2+has not been detected anatomically in retinal ganglion cells (Ugarte and Osborne, 1998), and Zn2+ that is bound to PKC would not be detectable by any anatomical methods that we are aware of (Frederickson, 1989; Sensi et al., 1997). However, one of the PKC isozymes (PKC-β) localized to retinal ganglion cells of the species used in this study (Osborne et al., 1992) is a type whose activity has been shown by biochemical methods to be zinc dependent (Murakami et al., 1987; Csermely et al., 1988; Hubbard et al., 1991). Our results provide independent evidence of such activity, and in the absence of other candidates, we provisionally attribute the PKC- and zinc-dependent regulation of Cl− current to this isozyme. This possibility could be of general interest because PKC-β has also been localized in frog, turtle, rabbit, monkey, and human retinal ganglion cells (Cuenca et al., 1990; Usuda et al., 1991;Osborne et al., 1992; Kolb et al., 1993; Fukuda et al., 1994).
Cl− current identification
Four types of Cl− current have been found previously to be reduced in amplitude by PKC activity. However, none of these currents are known to have all of the properties we report here. The Cl− current blocked by PKC activation in hippocampal pyramidal cells is activated by hyperpolarization and rectifies inwardly (Madison et al., 1986; Staley, 1994); large single-channel anion currents recorded after PKC inhibition or membrane patch excision (Kokubun et al., 1991; Groschner and Kukovetz, 1992) display bell-shaped activation curves; Cl− channels are inactivated by PKC in normal and cystic fibrosis airway epithelia, but only at cytoplasmic Ca2+ concentrations >150 nm (Li et al., 1989); and background Cl− currents that are reduced by PKC in other preparations [skeletal muscle (Tricarico et al., 1991); renal and cardiac ClC-3 channels (Kawasaki et al., 1994; Duan et al., 1997); and ciliary epithelium (Coca-Prados et al., 1995)] have yet to be examined for cytoplasmic Zn2+ sensitivity. Cytoplasmic Ca2+ inhibits ClC-3 channel currents (Kawasaki et al., 1995) at levels that did not noticeably alter theICl reported here. However, the significance of this difference remains to be investigated, because the inhibition by Ca2+ was observed under conditions unlike ours (in the presence of a protein kinase inhibitor and alkaline phosphatase, and in the absence of ATP and Zn2+) and because we have been unable to make stable whole-cell recordings from retinal ganglion cells in hypotonic or isotonic bath solutions (see Materials and Methods).
The background ICl we have found in retinal ganglion cells also appears to be distinct from all five types of Cl− current described in other retinal cells.ICl was not detectably affected by cytoplasmic Ca2+ levels buffered (nominally) to between 3 and 300 nm and thus differs from the Ca2+-activated Cl− current of photoreceptor and bipolar cells (see Results). In addition,ICl was not blocked by picrotoxinin and thus differs from Cl− channels complexed with GABAA, GABAC, and glycine receptors (see Wang et al., 1995). Lastly, IClwas not affected by Rp-cAMP and thus differs from the Cl− conductance in pigmented epithelial cells (Peterson et al., 1997). Coincidentally, protein kinase activities have been found to reduce the amplitude of sustained Cl−currents found so far in all retinal cells, regardless of the conditions under which they activate [resting voltage (Fig. 1) or exposure to GABA (Feigenspan and Bormann, 1994), glycine (Han and Slaughter, 1998), and cytoplasmic Ca2+ (Peterson et al., 1997)].
Cl− current function
Our results indicate that retinal ganglion cells possess at least two resting ion conductances. One is permeable to K+ions (Bindokas et al., 1994), whereas the other is the Cl− conductance reported here. Like the ohmic conductance recorded between −105 and −55 mV in the absence of extracellular K+ ions (Tabata and Ishida, 1996),ICl showed no time-dependent activation, inactivation, or deactivation. On the other hand, the relatively small density of ICl near resting potential and its nonlinear dependence on voltage are characteristic of background Cl− currents in various tissues (Franciolini and Petris, 1990). Regardless of its gating mechanism,ICl was consistently detected at test potentials as negative as −80 mV, suggesting that ICl is tonically activated in situ at membrane voltages at least as negative as the resting potential.
Because resting potential is relatively close to the K+ equilibrium potential, even if the Cl− equilibrium potential is raised to 0 mV (Ishida, 1995), K+ conductance evidently contributes more to resting potential than the Cl− conductance reported here (see also Ascher et al., 1976). However, our results imply that the contribution of ICl to resting potential and other electrical properties of cells (membrane time constant, input impedance, and synaptic integration) might not be fixed. Conceivably, these contributions could be augmented or diminished, given our observation that the Cl−permeability rises if recording conditions deplete cytoplasmic Zn2+ or inhibit PKC and that its resting level can be decreased by treatments that augment cytoplasmic Zn2+. Moreover, these contributions could be altered slowly and persistently by synaptic modulation of PKC activity, becauseICl showed no susceptibility to inactivation by membrane potential. Lastly, because we recordedICl at membrane potentials that are more negative and more positive than typical resting potentials, and because retinal ganglion cells are inhibited by GABA- and glycine-gated Cl− conductances in situ, a background Cl− permeability may provide retinal ganglion cells with a means of reducing the driving force for Cl−currents (and thus, of reducing membrane potential fluctuations) without obviating shunting types of inhibition. Until more is known about PKC regulation and the distribution of IClwithin retinal ganglion cells in situ, we refrain from further speculation about its possible functions.
Footnotes
This work was supported by National Institutes of Health Grant EY 08120 from the National Eye Institute (Bethesda, MD) to A.T.I. We thank Professor Philippe Ascher for helpful criticism of this manuscript, Professors Yutaka Fukuda and Masanobu Kano for the opportunity to pursue this study, and Ms. Gloria Partida for preparing the primary cell cultures used in these experiments.
Correspondence should be addressed to Dr. Andrew T. Ishida, Section of Neurobiology, Physiology, and Behavior, University of California, One Shields Avenue, Davis, CA 95616-8519.
Dr. Tabata’s present address: Department of Physiology, Kanazawa University School of Medicine, Kanazawa, Ishikawa 920–8640, Japan.
REFERENCES
- 1.Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. J Biol Chem. 1985;260:2719–2727. [PubMed] [Google Scholar]
- 2.Ascher P, Kunze D, Neild TO. Chloride distribution in Aplysia neurones. J Physiol (Lond) 1976;256:441–464. doi: 10.1113/jphysiol.1976.sp011332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol (Lond) 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begenisich T, Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974;63:675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bindokas VP, Yoshikawa M, Ishida AT. Na+-Ca2+ exchanger-like immunoreactivity and regulation of intracellular Ca2+ levels in fish retinal ganglion cells. J Neurophysiol. 1994;72:47–55. doi: 10.1152/jn.1994.72.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Brocard JB, Rajdev S, Reynolds IJ. Glutamate-induced increases in intracellular free Mg2+ in cultured cortical neurons. Neuron. 1993;11:751–757. doi: 10.1016/0896-6273(93)90084-5. [DOI] [PubMed] [Google Scholar]
- 7.Brooks SPJ, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 8.Burgoyne RD, Morgan A. Ca2+ and secretory-vesicle dynamics. Trends Neurosci. 1995;18:191–196. doi: 10.1016/0166-2236(95)93900-i. [DOI] [PubMed] [Google Scholar]
- 9.Callahan MJ, Korn SJ. Permeation of Na+ through a delayed rectifier K+ channel in chick dorsal root ganglion neurons. J Gen Physiol. 1994;104:747–771. doi: 10.1085/jgp.104.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang D, Hsieh PS, Dawson DC. Calcium: a program in BASIC for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Comput Biol Med. 1988;18:351–366. doi: 10.1016/0010-4825(88)90022-4. [DOI] [PubMed] [Google Scholar]
- 11.Coca-Prados M, Anguita J, Chalfant KL, Civan MM. PKC-sensitive Cl− channels associated with ciliary epithelial homologue of pICln. Am J Physiol. 1995;268:C572–C579. doi: 10.1152/ajpcell.1995.268.3.C572. [DOI] [PubMed] [Google Scholar]
- 12.Csermely P, Szamel M, Resch K, Somogyi J. Zinc can increase the activity of protein kinase C and contributes to its binding to plasma membranes in T lymphocytes. J Biol Chem. 1988;263:6487–6490. [PubMed] [Google Scholar]
- 13.Cuenca N, Fernandez E, Kolb H. Distribution of immunoreactivity to protein kinase C in the turtle retina. Brain Res. 1990;532:278–287. doi: 10.1016/0006-8993(90)91770-h. [DOI] [PubMed] [Google Scholar]
- 14.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 15.Erdahl WL, Chapman CJ, Wang E, Taylor RW, Pfeiffer DR. Ionophore 4-BrA23187 transports Zn2+ and Mn2+ with high selectivity over Ca2+. Biochemistry. 1996;35:13817–13825. doi: 10.1021/bi961391q. [DOI] [PubMed] [Google Scholar]
- 16.Etter EF, Minta A, Poenie M, Fay FS. Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18. Proc Natl Acad Sci USA. 1996;93:5368–5373. doi: 10.1073/pnas.93.11.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feigenspan A, Bormann J. Modulation of GABAc receptors in rat retinal bipolar cells by protein kinase C. J Physiol (Lond) 1994;481:325–330. doi: 10.1113/jphysiol.1994.sp020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes IJ, Zalewski PD, Giannakis C. Role for zinc in a cellular response mediated by protein kinase C in human B lymphocytes. Exp Cell Res. 1991;195:224–229. doi: 10.1016/0014-4827(91)90521-u. [DOI] [PubMed] [Google Scholar]
- 19.Franciolini F, Petris A. Chloride channels of biological membranes. Biochim Biophys Acta. 1990;1031:247–259. doi: 10.1016/0304-4157(90)90009-2. [DOI] [PubMed] [Google Scholar]
- 20.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda K, Saito N, Yamamoto M, Tanaka C. Immunocytochemical localization of the α, βI, βII- and γ-subspecies of protein kinase C in the monkey visual pathway. Brain Res. 1994;658:155–162. doi: 10.1016/s0006-8993(09)90021-x. [DOI] [PubMed] [Google Scholar]
- 22.Groschner K, Kukovetz WR. Voltage-sensitive chloride channels of large conductance in the membrane of pig aortic endothelial cells. Pflügers Arch. 1992;421:209–217. doi: 10.1007/BF00374829. [DOI] [PubMed] [Google Scholar]
- 23.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell free patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Slaughter MM. Protein kinases modulate two glycine currents in salamander retinal ganglion cells. J Physiol (Lond) 1998;508:681–690. doi: 10.1111/j.1469-7793.1998.681bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidaka S, Ishida AT. Voltage-gated Na+ current availability after step- and spike-shaped conditioning depolarizations of retinal ganglion cells. Pflügers Arch. 1998;436:497–508. doi: 10.1007/s004240050664. [DOI] [PubMed] [Google Scholar]
- 26.Holevinsky KO, Jow F, Nelson DJ. Elevation in intracellular calcium activates both chloride and proton currents in human macrophages. J Membr Biol. 1994;140:13–30. doi: 10.1007/BF00234482. [DOI] [PubMed] [Google Scholar]
- 27.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;94:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard SR, Bishop WR, Kirschmeier P, George SJ, Cramer SP, Hendrickson WA. Identification and characterization of zinc binding sites in protein kinase C. Science. 1991;254:1776–1779. doi: 10.1126/science.1763327. [DOI] [PubMed] [Google Scholar]
- 29.Ishida AT. Ion channel components of retinal ganglion cells. Prog Retin Eye Res. 1995;15:261–280. [Google Scholar]
- 30.Johnson BD, Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+. Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-d-aspartate-activated channels. Biophys J. 1990;57:1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki M, Suzuki M, Uchida S, Sasaki S, Marumo F. Stable and functional expression of the ClC-3 chloride channel in somatic cell lines. Neuron. 1995;14:1285–1291. doi: 10.1016/0896-6273(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 34.Kokubun S, Saigusa A, Tamura T. Blockade of Cl channels by organic and inorganic blockers in vascular smooth muscle cells. Pflügers Arch. 1991;418:204–213. doi: 10.1007/BF00370515. [DOI] [PubMed] [Google Scholar]
- 35.Kolb H, Zhang L, Dekorver L. Differential staining of neurons in the human retina with antibodies to protein kinase C isozymes. Vis Neurosci. 1993;10:341–351. doi: 10.1017/s0952523800003734. [DOI] [PubMed] [Google Scholar]
- 36.Lascola CD, Nelson DJ, Kraig RP. Cytoskeletal actin gates a Cl− channel in neocortical astrocytes. J Neurosci. 1998;18:1679–1692. doi: 10.1523/JNEUROSCI.18-05-01679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, McCann JD, Anderson MP, Clancy JP, Liedtke CM, Nairn AC, Greengard P, Welsh MJ. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989;244:1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- 38.Lin YF, Browning MD, Dudek EM, MacDonald RL. Protein kinase C enhances recombinant bovine α1-β1-γ2L GABA(A) receptor whole-cell currents expressed in L929 fibroblasts. Neuron. 1994;13:1421–1431. doi: 10.1016/0896-6273(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 39.Lukasiewicz PD, Werblin FS. A slowly inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci. 1988;8:4470–4481. doi: 10.1523/JNEUROSCI.08-12-04470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madison DV, Malenka RC, Nicoll RA. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986;321:695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- 41.Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron. 1988;1:503–515. doi: 10.1016/0896-6273(88)90181-x. [DOI] [PubMed] [Google Scholar]
- 42.Marks PW, Maxfield FR. Preparation of solutions with free calcium concentration in the nanomolar range using BAPTA. Anal Biochem. 1991;193:61–71. doi: 10.1016/0003-2697(91)90044-t. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- 44.Murakami K, Whiteley MK, Routtenberg A. Regulation of protein kinase C activity by cooperative interaction of Zn2+ and Ca2+. J Biol Chem. 1987;262:13902–13906. [PubMed] [Google Scholar]
- 45.Okada T, Horiguchi H, Tachibana M. Ca2+-dependent Cl− current at the presynaptic terminals of goldfish retinal bipolar cells. Neurosci Res. 1995;23:297–303. doi: 10.1016/0168-0102(95)00955-8. [DOI] [PubMed] [Google Scholar]
- 46.O’Rourke B, Backx PH, Marban E. Phosphorylation-independent modulation of L-type calcium channels by magnesium-nucleotide complexes. Science. 1992;257:245–248. doi: 10.1126/science.1321495. [DOI] [PubMed] [Google Scholar]
- 47.Osborne NN, Barnett NL, Morris NJ, Huang FL. The occurrence of three isozymes of protein kinase C (α, β and γ) in retinas of different species. Brain Res. 1992;570:161–166. doi: 10.1016/0006-8993(92)90577-v. [DOI] [PubMed] [Google Scholar]
- 48.Peterson WM, Quong JN, Blaug SA, Miller SS. Evidence that cyclic-AMP dependent chloride conductance is controlled by phospholamban rather than CFTR on bovine RPE. Invest Ophthalmol Vis Sci. 1997;38:s467. [Google Scholar]
- 49.Roberts WM. Spatial calcium buffering in saccular hair cells. Nature. 1993;363:74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- 50.Sekiguchi K, Tsukuda M, Ase K, Kikkawa U, Nishizuka Y. Mode of activation and kinetic properties of three distinct forms of protein kinase C from rat brain. J Biochem (Tokyo) 1988;103:759–765. doi: 10.1093/oxfordjournals.jbchem.a122343. [DOI] [PubMed] [Google Scholar]
- 51.Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh J-Y, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapiro MS, Zhou J, Hille B. Selective disruption by protein kinases of G-protein-mediated Ca2+ channel modulation. J Neurophysiol. 1996;76:311–320. doi: 10.1152/jn.1996.76.1.311. [DOI] [PubMed] [Google Scholar]
- 53.Smith RM, Martell AE. Critical stability constants. Plenum; New York: 1975. [Google Scholar]
- 54.Staley K. The role of an inwardly rectifying chloride conductance in postsynaptic inhibition. J Neurophysiol. 1994;72:273–284. doi: 10.1152/jn.1994.72.1.273. [DOI] [PubMed] [Google Scholar]
- 55.Stelzer A, Kay AR, Wong RKS. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988;241:339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- 56.Sucher NJ, Lipton SA. A slowly inactivating K+ current in retinal ganglion cells from postnatal rat. Vis Neurosci. 1992;8:171–176. doi: 10.1017/s0952523800009330. [DOI] [PubMed] [Google Scholar]
- 57.Tabata T, Ishida AT. Transient and sustained depolarization of retinal ganglion cells by Ih. J Neurophysiol. 1996;75:1932–1943. doi: 10.1152/jn.1996.75.5.1932. [DOI] [PubMed] [Google Scholar]
- 58.Tabata T, Ishida AT. Intracellular zinc may sustain PKC-mediated reduction of outwardly rectifying Cl− current in retinal ganglion cells. Soc Neurosci Abstr. 1997;23:2360. [Google Scholar]
- 59.Tricarico D, Conte Camerino D, Govoni S, Bryant SH. Modulation of rat skeletal muscle chloride channels by activators and inhibitors of protein kinase C. Pflügers Arch. 1991;418:500–503. doi: 10.1007/BF00497778. [DOI] [PubMed] [Google Scholar]
- 60.Ugarte M, Osborne NN. The localization of endogenous zinc and the in vitro effect of exogenous zinc on the GABA immunoreactivity and formation of reactive oxygen species in the retina. Gen Pharmacol. 1998;30:297–303. doi: 10.1016/s0306-3623(97)00358-3. [DOI] [PubMed] [Google Scholar]
- 61.Usuda N, Kong Y, Hagiwara M, Uchida C, Terasawa M, Nagata T, Hidaka H. Differential localization of protein kinase C isozymes in retinal neurons. J Cell Biol. 1991;112:1241–1247. doi: 10.1083/jcb.112.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T-L, Hackam AS, Guggino WB, Cutting GR. A single amino acid in γ-aminobutyric acid ρ1 receptors affects competitive and noncompetitive components of picrotoxin inhibition. Proc Natl Acad Sci USA. 1995;92:11751–11755. doi: 10.1073/pnas.92.25.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woll KH, Leibowitz MD, Neumcke B, Hille B. A high-conductance anion channel in adult amphibian skeletal muscle. Pflügers Arch. 1987;410:632–640. doi: 10.1007/BF00581324. [DOI] [PubMed] [Google Scholar]
- 64.Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using Zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid] a new specific fluorescent probe for Zn(II). Biochem J. 1993;296:403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y, Ikeda SR. Anomalous permeation of Na+ through a putative K+ channel in rat superior cervical ganglion neurones. J Physiol (Lond) 1993;468:441–461. doi: 10.1113/jphysiol.1993.sp019781. [DOI] [PMC free article] [PubMed] [Google Scholar]