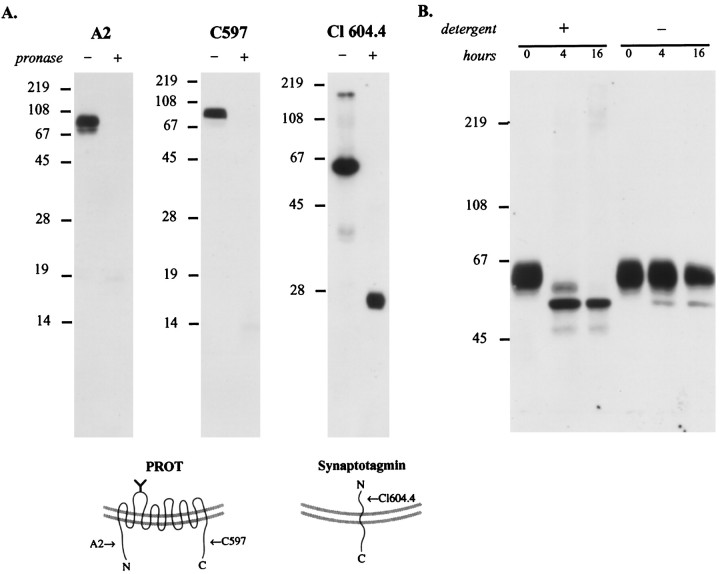
Fig. 8.
Mapping the topology of PROT in the synaptic vesicle membrane. A, PROT N and C termini are oriented cytoplasmically. The location of PROT termini was mapped by subjecting a synaptic vesicle-enriched fraction (LP2) to limited proteolysis. After a 20 min incubation with or without Pronase the samples were separated by SDS-PAGE (15 or 12%) and subjected to immunoblot analysis by using domain-specific antibodies. PROT immunoreactivity is present only in the Pronase-negative reaction when probed with antibodies specific for the N terminus (A2; 1:10,000 dilution) or C terminus (C597; 1:40,000 dilution), indicating that these structures are present on the external (cytoplasmic) face of the vesicle membrane. In contrast, a monoclonal antibody against the intraluminal N-terminal domain of the integral synaptic vesicle protein synaptotagmin (Cl 604.4; 1:1000 dilution) recognized the protected epitope in the partially digested protein. This indicates that during the protease reaction the synaptic vesicles were intact, and loss of PROT immunoreactivity was not attributable to vesicle rupture. The arrows in the models indicate the positions of the epitopes of the antibodies used for immunoblotting.B, The N-glycosylated loop is intraluminal. A synaptic vesicle-enriched fraction, LP2, was subjected to deglycosylation by PNGase F in the presence or absence of detergents (2.5% NP-40/1% SDS), separated by SDS-PAGE (8%), and immunoblotted with anti-PROT antibody C597 (1:40,000 dilution). In the presence of detergent the native PROT protein is reduced progressively to a single band of ∼53 kDa. However, in the absence of detergent a significant loss of glycosylation fails to occur, indicating that the N-linked glycosylation site is located within the vesicle lumen.