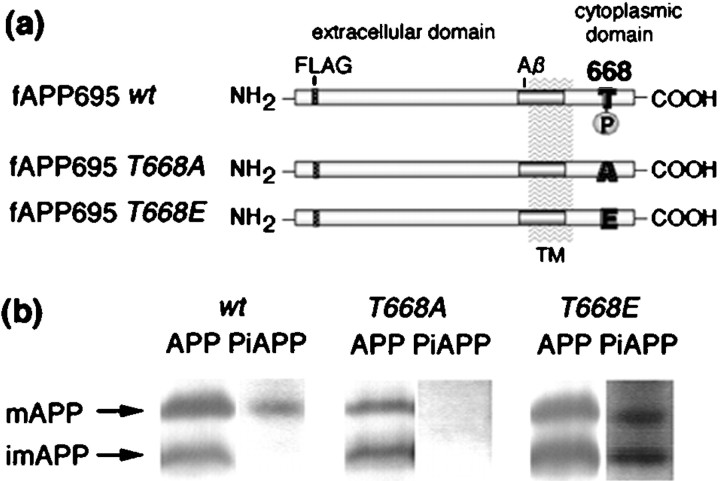
Fig. 3.
Domain organization of wild-type and mutant fAPP695 molecules and analysis of their expression and phosphorylation in PC12 cells. a, The domain organization of fAPP695 is illustrated. The positions of the FLAG sequence (FLAG), the transmembrane domain (TM), the β-amyloid (Aβ) domain, and the phosphorylation site, T668 (668), are indicated. The mutation of Thr668 is also indicated: in fAPP695T668A, threonine is replaced by alanine; in fAPP695T668E, threonine is replaced by glutamate. b, APP was immunoprecipitated from cell lysates (1.5 mg of protein) from PC12 cells stably expressing fAPP695wt (wt), fAPP695T668A (T668A) or fAPP695T668E (T668E) using M2 (FLAG) antibody, and the samples were subjected to SDS-PAGE [6% (w/v) polyacrylamide]. Samples were analyzed by Western blot using UT-421 (APP) and UT-33 (PiAPP) antibodies. Immunocomplexes were detected with 125I-protein A, and APP and PiAPP were quantified using a Fuji BAS 2000 Imaging Analyzer.mAPP, Mature fAPP695; imAPP, immature fAPP695.