Abstract
Odor coding relies on the activity of different classes of receptor neurons, each with distinct response characteristics. We have examined odor coding in a model olfactory organ, the maxillary palp ofDrosophila. This organ contains only 120 olfactory receptor neurons, compartmentalized in sensory hairs called sensilla, and provides an opportunity to characterize all neurons in an entire olfactory organ. Extensive extracellular recordings from single sensilla reveal that the neurons fall into six functional classes. Each of the 60 sensilla houses two neurons, which observe a pairing rule: each sensillum combines neurons of two particular classes, thereby yielding three sensillum types. The sensillum types are intermingled on the surface of the palp, but their distribution is not random. The neurons exhibit diverse response characteristics, providing the basis for an olfactory code. A particular odor can excite one neuron and inhibit another, and a particular neuron can be excited by one odor and inhibited by another. Some excitatory responses continue beyond the end of odor delivery, but responses to most odors terminate abruptly after the end of odor delivery, with some followed by a period of poststimulus quiescence. The specificity of odor response is examined in detail for the neurons of one sensillum, which were found to differ in their relative responses to a homologous series of esters. Adaptation and cross-adaptation are documented, and cross-adaptation experiments demonstrate that the two neurons within one type of sensillum can function independently. The analysis of all neuronal types in this model olfactory organ is discussed in terms of its functional organization and the mechanisms by which it encodes olfactory information.
Keywords: Drosophila, olfaction, maxillary palps, odor coding, single-unit electrophysiology, sensory field, adaptation, inhibition
Olfactory systems detect and differentiate among many kinds of odor stimuli. Olfactory receptor neurons encode qualitative, quantitative, temporal, and spatial information about odors. Elucidating the mechanisms by which olfactory information is encoded is an intriguing problem in contemporary neurobiology (Derby and Ache, 1984; Buck, 1996; Hildebrand and Shepherd, 1997). A major difficulty has been the complexity of most olfactory systems. In the case of mammals, for example, the number of olfactory receptor neurons (ORNs) is very large, as is the number of distinct functional classes into which these neurons may fall (Buck and Axel, 1991). Moreover, in many systems it is difficult to measure systematically the physiological properties of individual ORNs in vivo (Sicard and Holley, 1984).
Insect ORNs are distributed in sensilla, usually in the form of sensory hairs that protrude from the cuticle, providing accessibility and ease of identification (Altner and Prillinger, 1980; Boeckh, 1981). Odorants pass through tiny pores in the walls of these sensilla and stimulate dendrites bathing in the lymph inside. Most sensilla are on the antenna, and elegant analyses have characterized their physiological properties in moths, cockroaches, and other insects with large antennae (Boeckh et al., 1987; Hansson, 1995).
The fly Drosophila melanogaster has a relatively simple olfactory system. Olfactory response can be measured in vivo, via either physiological or behavioral means, and a variety of genetic and molecular approaches are available to study its olfactory system (Siddiqi, 1991; Carlson, 1996). There are only 1200 ORNs in the Drosophila antenna, housed in three morphologically distinct sensillum categories: trichoid, coeloconic, and basiconic sensilla (Stocker, 1994) (Fig.1A). Electrophysiological recordings from each of these categories indicate that they can be subdivided into different functional types (Siddiqi, 1991; Clyne et al., 1997), but an exhaustive description is not available.
Fig. 1.
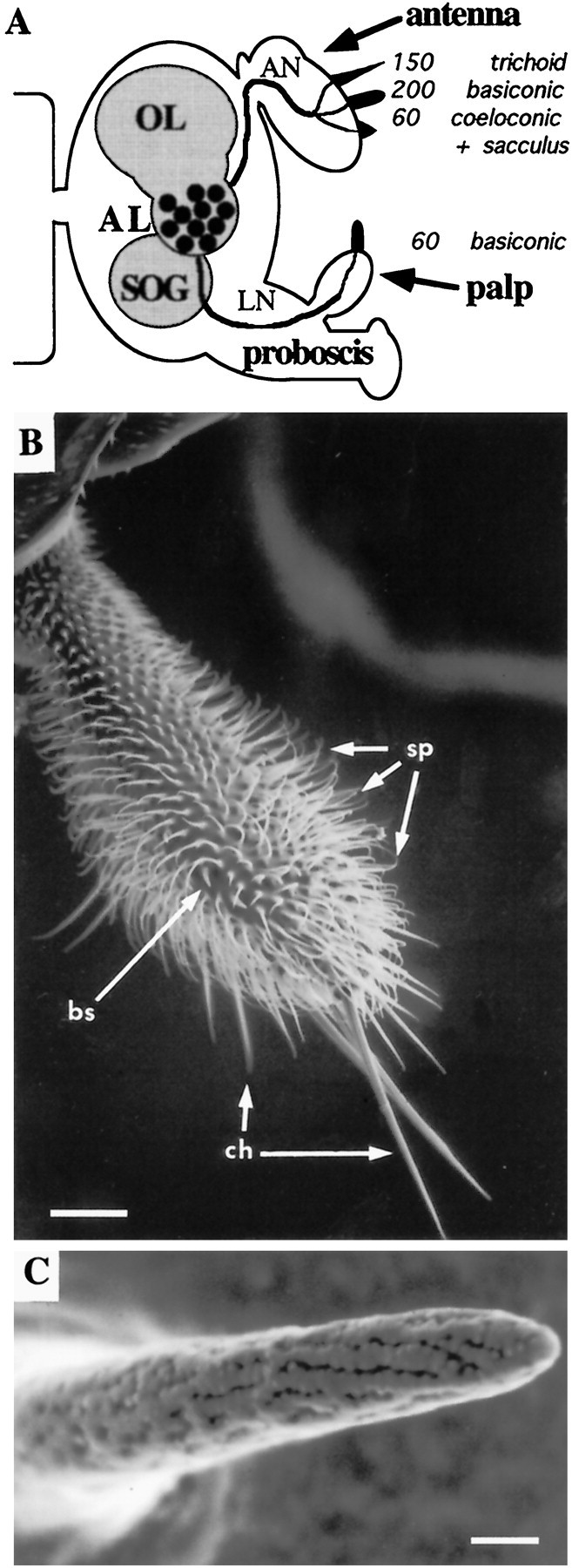
The maxillary palps carry olfactory sensilla.A, Schematic overview of the Drosophilaolfactory system showing three structurally different sensillum types and their numbers on two olfactory organs as well as their projections to the CNS. Sensilla in the sacculus, a multichambered sensory pit, are not enumerated. AL, Antennal lobe; AN, antennal nerve; LN, labiomaxillary nerve;OL, optic lobe; SOG, subesophageal ganglion. B, Scanning electron micrograph of the maxillary palp showing three types of cuticular hairs: olfactory sensilla (bs, sensilla basiconica), mechanosensory setae (ch, sensilla chaetica), and uninnervated hairs (sp, spinules). Scale bar, 25 μm. C, Scanning electron micrograph detail of a basiconic sensillum showing a multitude of pores through which odorants may pass. Scale bar, 1 μm.B and C are reprinted with permission from Riesgo-Escovar et al. (1997).
Even simpler than the antenna is the maxillary palp, an olfactory organ that extends from the proboscis and that contains an order of magnitude fewer neurons than does the antenna (Fig.1B,C) (Singh and Nayak, 1985). Each of the paired maxillary palps contains only 120 ORNs, housed in 60 sensilla of a single category, sensilla basiconica. The sensitivity of the Drosophila maxillary palp to a variety of odors has been demonstrated by field recordings (Ayer and Carlson, 1992). The numerical simplicity of the maxillary palp makes it possible to perform an exhaustive study of its neuronal composition and to characterize in detail its functional organization.
In this article we provide an extensive description of the olfactory receptor neurons of the maxillary palp, a simple model olfactory organ. Using single-unit recordings (Kaissling, 1995) and a panel of test odorants, we characterize the response profiles of the neurons and find that they fall into distinct functional classes, with different classes presumably reflecting distinct receptor–ligand interactions. We consider the issue of how narrowly some of the neurons are tuned. The neurons are shown to be housed in stereotyped pairs in three functional types of sensilla, whose distributions are characterized. Physiological analysis of the neurons reveals a diversity of response mechanisms. We also document adaptation and cross-adaptation of these neurons, and via cross-adaptation experiments, we find evidence that the two neurons that cohabit in a sensillum can function independently.
MATERIALS AND METHODS
Single-sensillum recordings. All flies were Canton-S wild-type (CS-5) and were reared at 18°C on standard yeasted corn meal–molasses medium (Helfand and Carlson, 1989). Electrophysiological recordings were done at 20°C and 40–60% relative humidity. A male fly (2–10 d old) was wedged into the narrow end of a truncated plastic pipette tip and placed on a slide under an Olympus BX40 (1000×) microscope (Olympus Optical, Tokyo, Japan). The proboscis was exposed and stuck to the pipette tip with a small piece of tape at the labellum. The distal end of the maxillary palp was held and stabilized by the broken tip of a tapered glass microcapillary tube. Recordings of action potentials were made by inserting a tungsten wire electrode into the base of a sensillum on the palp (Fig.2). The tungsten wire (0.1 mm diameter; General Electric, Cleveland, OH) was electrolytically sharpened (∼1 μm tip diameter) by dipping it repeatedly in a 10% NaNO2solution while passing a 0.3–3 mA current. The reference electrode was inserted more proximally in the proboscis, and signals were amplified 1000× (iso-dam; World Precision Instruments, Sarasota, FL) and fed into a computer via a 16-bit analog-to-digital converter to be analyzed off-line with AUTOSPIKE software (Syntech, Hilversum, The Netherlands).
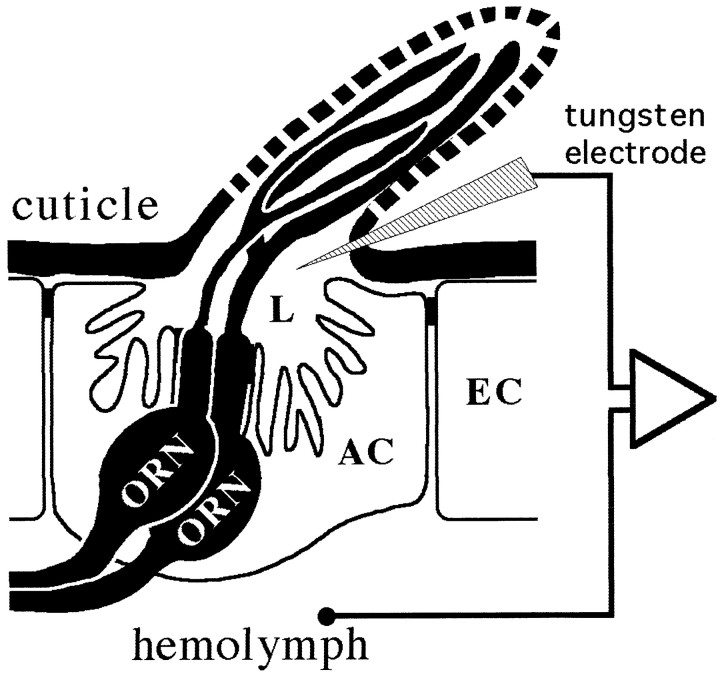
Fig. 2.
Schematic overview of single-unit recordings from ORNs in a basiconic sensillum, showing electrode positions for the extracellular recording of voltage differences between the sensillum lymph (L) and the hemolymph. AC, Accessory cells; EC, epidermal cells.
AC signals (100–10,000 Hz) were recorded for 6 sec, starting 2 sec before stimulation, and action potentials were counted off-line in a 500 msec period before stimulation and during the 500 msec stimulation. Responses of individual neurons were then calculated as the increase (or decrease) in action potential frequency (spikes per second). In addition, action potentials were extracted by computer using an AUTOSPIKE algorithm that distinguishes their peak-to-trough amplitudes from noise (Anderson et al., 1995; Taneja and Guerin, 1997). This was done either off-line from the primary data (see Fig. 3A) or on-line to monitor the frequency of an individual neuron for up to 60 sec (see Fig. 11A,C).
Fig. 3.
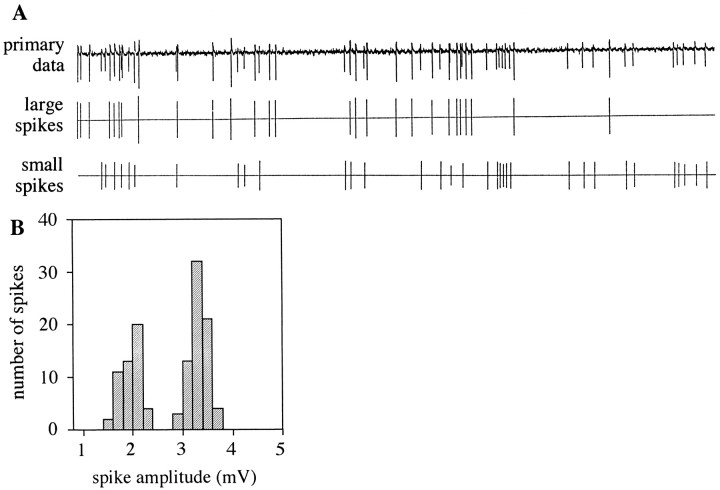
Single-unit recordings from palpal basiconic sensilla confirm the presence of two olfactory receptor neurons.A, Differences in spike amplitudes allow separate analysis of firing rates of the two neurons in a single sensillum.Top, Primary data from a 3 sec period of spontaneous activity from a sensillum are shown. Middle, bottom, The extracted data are presented with large spikes shown separately from small spikes. B, The distribution of amplitudes of individual action potentials (measured from peak-to-trough) is bimodal. Data shown are for 123 spikes from 6 sec of spontaneous activity of the recording in A.
Fig. 11.
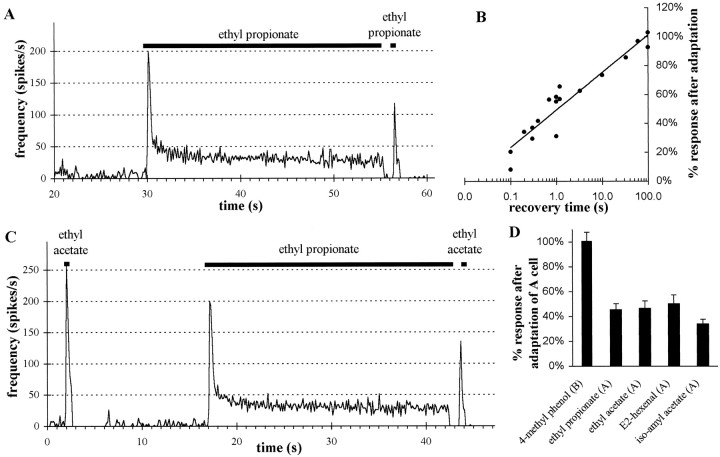
Adaptation and cross-adaptation in pb1 sensilla show convergence of olfactory information in pb1A neurons but independence of two neurons in a sensillum. A, Frequency of action potentials (averaged over 120 msec intervals) during prolonged exposure of pb1A to ethyl propionate is shown. The long stimulation (25 sec) was generated using the flask flow method (10−2 dilution), whereas the ensuing short stimulus (500 msec) was delivered with the syringe puff method (10−3.5 dilution; see Materials and Methods). Both stimulations elicit the same spike frequency during the first 500 msec from unadapted neurons. Note that frequencies over 500 msec are not the same as peak responses averaged over 120 msec. B, The response to the second stimulation with ethyl propionate increases, approximately following a linear function of the logarithm of the recovery time after adaptation. Responses are presented as a percentage of the response to an identical 500 msec stimulus given before adaptation. C, The pb1A neuron cross-adapts to ethyl acetate, after stimulation with ethyl propionate. A 10−2 dilution of ethyl acetate and a 10−3.5 dilution of ethyl propionate were used, both using the syringe puff method, because they elicit the same response from pb1A (see Fig. 9). D, The pb1A neuron was adapted to ethyl propionate as in A. Then the response was measured from pb1B to 4-methylphenol (10−2dilution; n = 13) or from pb1A to ethyl propionate (10−3.5 dilution; n = 10), ethyl acetate (10−2 dilution; n= 8), E2-hexenal (10−1.5 dilution;n = 8), or isoamyl acetate (10−1 dilution; n = 7). All the doses used were determined from Figure 9 to give approximately equal responses before adaptation. Error bars indicate SEM. Cross-adaptation is observed for all odorants exciting pb1A but not for 4-methylphenol, which stimulates pb1B.
Odor stimulation. A glass tube held 8 mm from the preparation continuously supplied humidified air to the preparation (35 ml/sec giving an airspeed of 180 cm/sec). For reliably delivering odor puffs using many odorants without cross-contamination, we used the headspace from 5 ml disposable syringes. A 2 ml/sec flow of nitrogen entered the airstream from a needle connected to an empty syringe inserted into a small hole in the glass tube at a distance of 85 mm from the preparation. Both air and nitrogen were ultrapure grade (Airgas, Cheshire, CT) and were cleaned over a charcoal filter. A second needle, inserted through the same hole, was connected to a syringe containing a small piece of filter paper laden with the odorant dissolved in 20 μl of paraffin oil at a 10−2dilution (unless otherwise indicated). The nitrogen stream could be switched for a brief stimulation period from the empty syringe to the odor-filled syringe by a solenoid valve expelling part of the headspace into the airstream. Thus, all elements of the airstream were kept constant except for a brief pulse of odor. A 500 msec stimulus period was used in all recordings using this “syringe puff” method.
A series of odors was administered three times, with the odors presented one after the other and an interval of at least 60 sec between the delivery of each odor. For structure–response studies with a series of nine esters, the order of presentation was randomized. For dose–response relations, odorants were presented with increasing doses in log or half-log steps. All odors were from Aldrich (Milwaukee, WI) and of the highest grade available (97–99%) exceptcis-vaccenyl acetate (Z11-octadecenyl acetate), which was from Sigma (99%; St. Louis, MO). For α-pinene, we used theR (+) isomer, and 4-methylcyclohexanol was a mix ofcis and trans isomers. The paraffin oil diluent was IR-spectroscopy grade (Fluka, Buchs, Switzerland)
Cluster analysis. An objective classification of data sets into classes of neurons was made with a hierarchical cluster analysis (Anderberg, 1973; Derby and Ache, 1984; Bieber and Smith, 1986) using JMP software (SAS Institute, Cary, NC). This method organizes a data set into discrete clusters on the basis of a number of variables describing each data point. In the case of ORNs, the variables we based the classification on are the responses to a set of five odorants (see Fig. 7). With the responses to each odorant as parameters, each neuron’s profile is a point in a five-dimensional space. An iterative process then groups the points into clusters on the basis of their distances, starting with the closest points, until all are grouped. The distances were calculated according to Ward’s method, which minimizes variance (i.e., ANOVA sum of squares) within clusters (Ward, 1963; Getz and Akers, 1997). The number of clusters that best represents the organization of the data is determined with the scree procedure (Bieber and Smith, 1986). This procedure identifies a sharp increase in the distances among clusters, indicating a change in the nature of the clustering steps.
Fig. 7.
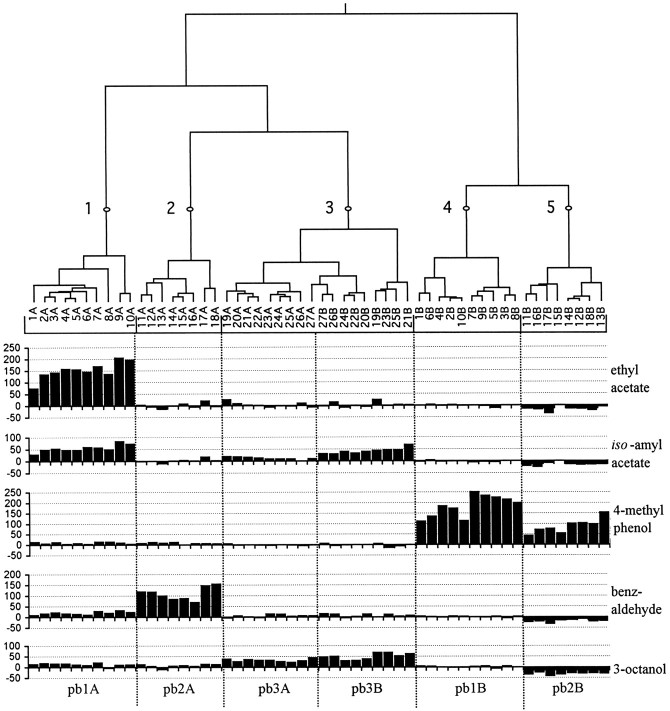
Dendrogram of a hierarchical cluster analysis of 54 ORNs in 27 sensilla, comparing their responses to five odors. Branch length is proportional to distance (see Materials and Methods), and five clusters are indicated by the numbered points. Individual ORNs are indicated as the A or B cell of a single sensillum, and their responses, measured as the increase in spikes per second over the spontaneous frequency (y-axis), are shown. Six classes of neurons are indicated at the bottom of the figure. Clusters 1, 2, 4, and 5 correspond to pb1A, pb2A, pb1B, and pb2B, respectively; cluster 3 includes two cell types within the pb3 sensillum, neurons pb3A and pb3B, that can be clearly distinguished by their response to other odors (see Fig. 8) and by differences in spike amplitude (data not shown).
Adaptation experiments. For adapting ORNs to continuing stimulation with one particular odorant, we needed to ensure that stimulus intensity stayed constant during a long stimulation period. The syringe puff method only allows reliable delivery of small samples from 5 ml of odor-saturated air. Instead we established a dynamic equilibrium between ethyl propionate, diluted 10−2in 200 μl of paraffin oil applied to a filter paper on the inside wall of a 15 ml gas-wash flask, and a continuous air flow (2 ml/sec). A solenoid valve then sent this odor-laden air via a needle either into the airstream toward the preparation or into a vacuum line to prevent odor buildup in the room. The equilibrium in this “flask flow” method was established during a period of at least 3 min before stimulation, and a single flask was not used for >20 min. The response of the two cells in one sensillum was recorded during a 500 msec stimulation with the syringe puff method. The sensillum was then stimulated for 25 sec with the flask flow method, and 1 sec after this, the response to a second 500 msec odor puff identical to the first was again recorded.
RESULTS
Olfactory receptor neurons of the palp show excitatory and inhibitory responses
ORNs from single sensilla on the Drosophila maxillary palp exhibited spontaneous action potentials, clearly distinguishable from background noise. The spikes from an individual sensillum could be resolved into two distinct populations, based on their amplitudes. A recording from a typical sensillum is shown in Figure3A, with the spikes of each population, large and small, indicated separately below the primary data. This bimodal distribution of spike amplitudes is illustrated in Figure 3B. We interpret the two populations of spikes as representing the activities of two distinct neurons, an interpretation that has been extensively supported in a wide variety of other insect sensory systems (Loftus and Corbière-Tichané, 1981;Fujishiro et al., 1984; Hansson et al., 1994; Anderson et al., 1995;Getz and Akers, 1997; Taneja and Guerin, 1997). In the case of theDrosophila maxillary palp, the presence of two neurons has been independently demonstrated in an ultrastructural analysis by Singh and Nayak (1985), who found two sensory cells with branched dendrites in individual basiconic sensilla. We will refer henceforth to the two neurons of a sensillum as the A and B cells.
Diversity among the olfactory neurons of the maxillary palp can be observed by analysis of their spontaneous firing rates. The spontaneous action potential frequency of individual cells varies, with most neurons exhibiting frequencies between 3 and 13 spikes/sec. The spontaneous rate of one of these individual cells is relatively constant, however; it stayed within a range of ±3 spikes/sec over the course of recording periods lasting as long as 60 min. In addition to these neurons, some neurons had noticeably higher spontaneous rates of ∼30 spikes/sec (see Fig. 6).
Fig. 6.
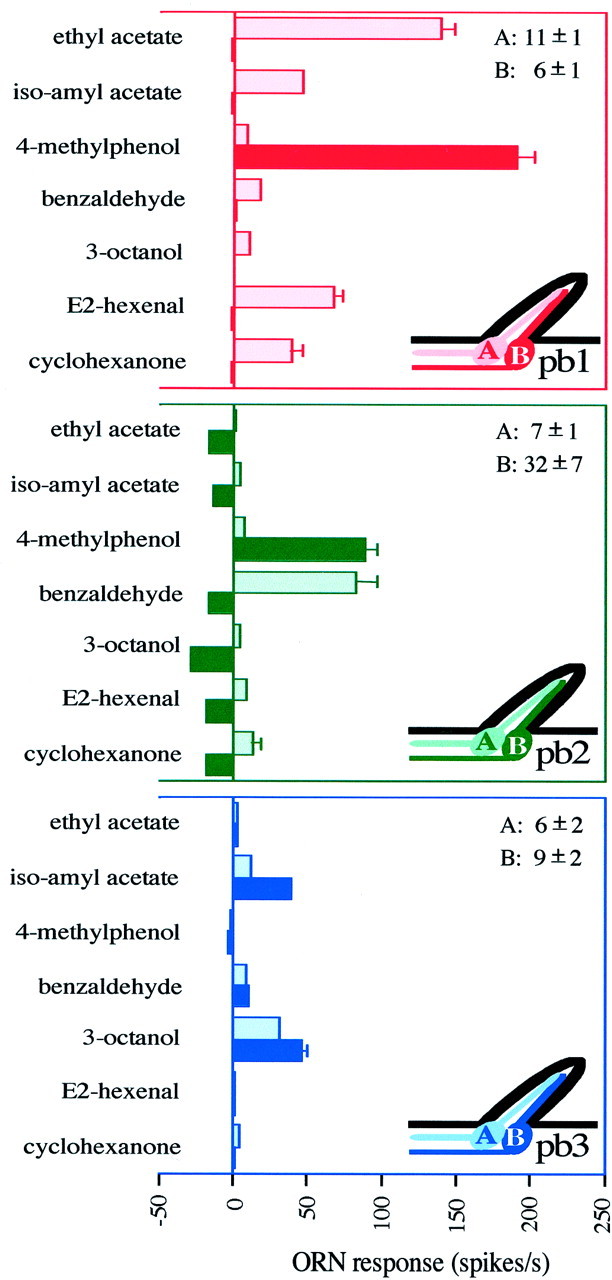
Six classes of olfactory receptor neurons are found in characteristic pairs (A and B neurons) in three functional types of sensilla on the Drosophila maxillary palp. Response profiles of ORNs are shown for the three sensillum types: pb1 (n = 17), pb2 (n = 15), and pb3 (n = 14). The spontaneous frequencies of the two neurons (± SD) are indicated in the top right corner for each type. The ORN response is measured as the increase (or decrease) in spikes per second over the spontaneous frequency. Error bars indicate SEM and are too small to be seen in some cases.
Odor stimulation elicited a marked increase in firing frequency in many cases (Fig. 4). In most cases one of the two neurons in a sensillum was clearly more excited than was the other by stimulation with a particular odor. The excited neuron can generally be identified by its spike height at the start of the response. For example, in Figure 4A only the A cell, that with greater amplitude, shows an increased frequency of firing in response to odor stimulation. The B cell, whose smaller spikes are indicated bydots, shows no excitation. In some cases the spike amplitude of the responding neuron gradually changes during the course of stimulation, a phenomenon widely observed in single-sensillum recordings from insects (discussed in Guillet and Bernard, 1972;Fujishiro et al., 1984; Rumbo, 1989). In such cases, the identities of the neurons can often be confirmed if necessary by examination of the spike shapes, which differ between the two neurons. Excitatory responses started ∼80 msec after odor was administered; calculations reveal that 50 msec is required for odor to reach the preparation, indicating a response latency of ≤30 msec. The spike frequency rose to a maximum within 50–100 msec after response initiation, depending on the odor and dose, and then declined as described in detail below.
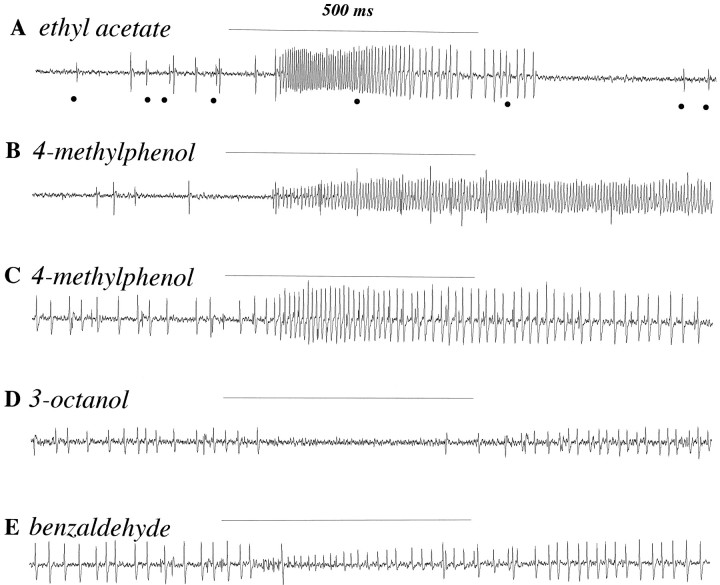
Fig. 4.
Sensilla house receptor neurons with a variety of different response characteristics to different odors.A–E, Five 1500 msec traces of recordings from two different sensilla showing excitatory and inhibitory responses of the two cells to 500 msec stimulations (horizontal lines) with different odorants. For odor stimulation, air was expelled from 5 ml syringes over filter paper laden with 20 μl of odorant. The odorants were diluted 10−2 in paraffin oil. We do not know the exact concentrations of the odor present in the air reaching the preparation. A, B, Recordings from one sensillum, later classified as pb1. Large action potentials, from the A neuron, increase their frequency in response to ethyl acetate. Dots indicate smaller action potentials from the B neuron, which is not excited by ethyl acetate but which responds to 4-methylphenol. C–E, Recordings from another sensillum, later classified as pb2. Large spikes are from the B neuron, which is excited by 4-methylphenol and inhibited by other odors. Smaller spikes are from the A neuron, excited by benzaldehyde.
Excitatory responses terminated abruptly after the end of the odor stimulation period in most cases, such as that shown in Figure4A. This is consistent with the expected sharp decline in odor levels after the 500 msec delivery period. In these cases we often observed a brief period in which no action potentials were recorded from this neuron. The duration of this poststimulus quiescence appeared to be dose-dependent, with higher doses producing longer periods of quiescence (data not shown), although we have not examined this relationship quantitatively. Poststimulus quiescence was not, however, observed for all odors. In fact, in some cases excitation continues long past the end of odor stimulation; Figure4B shows a recording from the same sensillum analyzed in Figure 4A but stimulated with 4-methylphenol instead of ethyl acetate. In this case stimulation continues past the end of the odor delivery period and declines to spontaneous levels over the course of several seconds. The same stimulus elicits a similar response, of smaller magnitude, from a different sensillum and again shows a prolonged pattern of firing (Fig. 4C). Thus the variations in response at the end of odor stimulation show that different odors may have different effects on the kinetics of excitation, thereby providing one putative mechanism for odor discrimination.
Inhibitory responses were also observed in some sensilla. Figure4D shows inhibition of firing in one neuron of such a sensillum after stimulation with 3-octanol. Moreover, this neuron can be excited by one odor and inhibited by others. For example, the neuron shown to be inhibited by 3-octanol in Figure 4D is the same one excited by 4-methylphenol in Figure 4C. Inhibition was observed only in those cells that showed the higher rate of spontaneous action potentials (30 spikes/sec). Interestingly, in these sensilla some odors inhibit one cell but excite the other (Fig.4E); the cell with the large spikes is inhibited, and the cell with the small spikes is excited. Thus an individual odor can have opposite effects on the firing frequency of different neurons, and an individual neuron can respond oppositely to different odors.
The olfactory neurons fall into six functional classes and are housed in stereotyped pairs within three sensillum types
To define the basic elements of the olfactory code, we sought to determine whether ORNs fall into discrete functional classes and, if so, to determine the number and odor specificity of such classes. We initiated this analysis with a chemically diverse group of 16 odorants selected on the basis of three criteria. Some odorants (ethyl acetate, 3-octanol, and benzaldehyde) were selected because they had been used extensively in previous research on Drosophila olfaction and are known to induce strong behavioral responses. Others were selected because they play important roles in the ecology of related dipteran insects. For example, 4-methylphenol is present in cattle urine and attracts tsetse flies to their hosts (Bursell et al., 1988); E2-hexenal (leaf aldehyde) is a plant odor that attracts many insect species (Visser, 1986). Finally, odors were selected to represent certain chemical groups (e.g., ketones, aldehydes, alcohols, esters, and aromatics).
Our initial recordings from individual sensilla clearly indicated that sensilla could be divided into three functional types, which we have termed palpal basiconic 1 (pb1), pb2, and pb3. Although of the same morphological category, the three types of sensilla contain neurons with response spectra that are distinct from each other and from neurons of the other sensillum types. Figure5 shows responses to the 16 test odors recorded from the two neurons of the pb1 sensillum, neurons that we denote pb1A and pb1B. The pb1B cell responds strongly to only one of the tested odorants, 4-methylphenol, to which it shows an increase in spike frequency of 178 ± 47 spikes/sec (± SD; n= 13). The only other response from pb1B that was significantly different from that of the paraffin oil control is the response to 4-methylcyclohexanol, an odor molecule structurally similar to 4-methylphenol. The pb1A cell, by contrast, shows a broader range of responses to the tested stimuli; it responds most strongly to ethyl acetate, showing an increase of 138 ± 32 spikes/sec (± SD;n = 13), but also responds to several other stimuli.
Fig. 5.
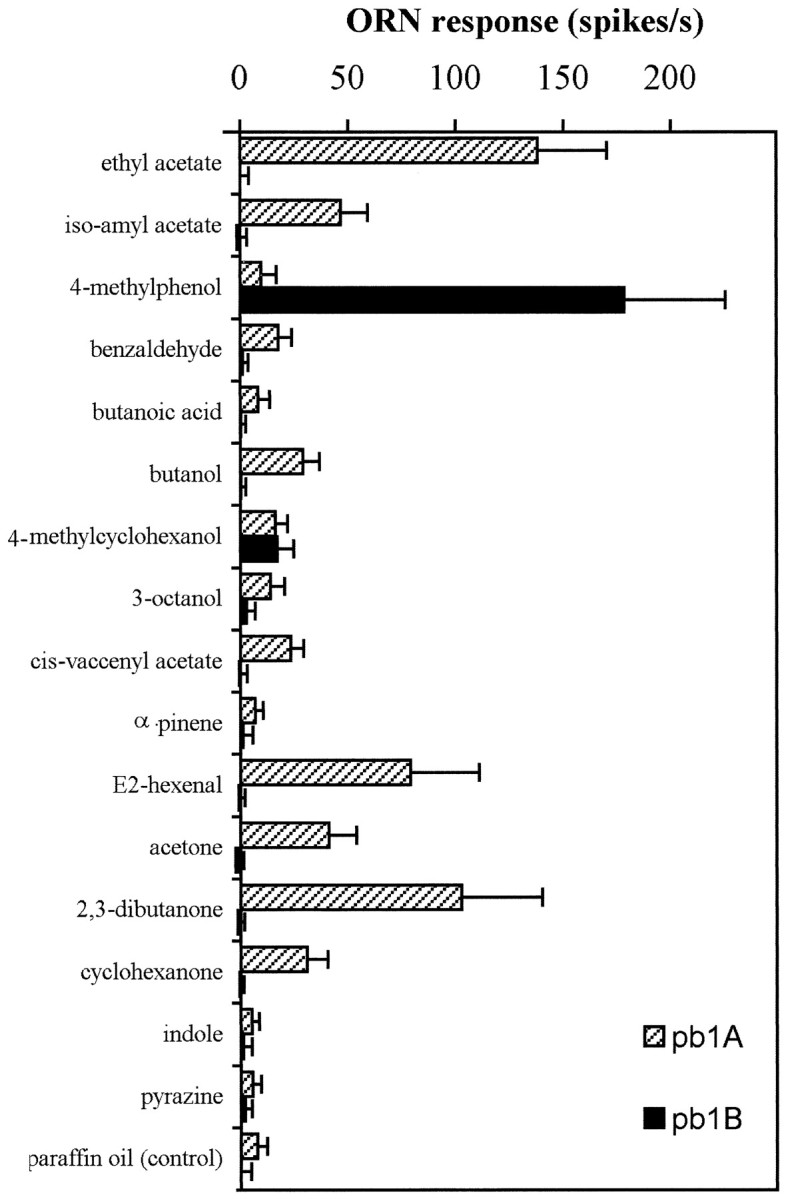
Responses of the two pb1 neurons to a set of 16 odorants (error bars indicate SD; n = 13). pb1B responds strongly only to one of the tested odors. Responses of pb1A to several odorants are significantly larger than the control response to the paraffin oil diluent alone. The indicated ORN response is measured as the increase in spikes per second over the spontaneous frequency.
The different neurons of the maxillary palp could be distinguished using a diagnostic subset of 7 of the 16 odors. We therefore used these 7 odors to extend our analysis to a larger number of sensilla. The results confirm the presence of exactly three sensillum types, each containing two neurons, yielding a total of six types of neurons with distinguishable response properties: pb1A, pb1B, pb2A, pb2B, pb3A, and pb3B (Fig. 6). The pb1 sensilla contain an A cell that responds strongly to ethyl acetate and a B cell that responds strongly to 4-methylphenol. In the pb2 sensilla, the A cell responds strongly to benzaldehyde. The other cell, pb2B, is excited by 4-methylphenol, although not as strongly as is pb1B. In addition, pb2B is strongly inhibited by 3-octanol and several other odors. Specifically, the spontaneous firing frequency of pb2B is 32 ± 7 spikes/sec and is reduced 80–100% by 3-octanol. The pb3 sensillum contains two neurons that are both excited by 3-octanol and isoamyl acetate, but pb3B is more strongly stimulated by isoamyl acetate than is pb3A. In summary, this analysis revealed that the maxillary palp contains six distinguishable neuronal types. The responses of some neuronal types are overlapping (e.g., 4-methylphenol excites both pb1B and pb2B neurons), but comparisons of responses to multiple odors clearly showed the profiles to be distinct. We note finally that in pb1 and pb3 sensilla, A neurons consistently have larger spike amplitudes than B neurons. pb2 sensilla differ from pb1 and pb3 sensilla not only in that the B neuron consistently has higher spontaneous activity than the other five neuronal types but also in that pb2A has smaller spikes than pb2B in some sensilla. However, spikes of pb2A are consistently different in shape, having larger negative phases relative to the positive phase.
The two neurons in one sensillum observe a pairing rule; for example, the cell that is excited by benzaldehyde (pb2A) is always paired with a cell that is inhibited by 3-octanol (pb2B). Among 232 sensilla examined, we recorded the activity of two cells in 225 cases (in the 7 exceptional sensilla, one cell was absent or unresponsive, perhaps because of damage caused by the recording electrode in at least some cases); in 222 of the 225 cases, we found one of the three characteristic combinations of cells.
Coding of odor quality and quantity across cell classes
Although the existence of exactly six neuronal classes seemed clear from this physiological analysis (Fig. 6), we sought to test our classification scheme more rigorously. We therefore performed a cluster analysis of the responses of 54 neurons in 27 sensilla to five of the odors (Fig. 7). This analysis yielded five clusters based on the response profiles. Cluster 1 groups cells with a strong response to ethyl acetate (corresponding to pb1A), and cluster 2 unites cells responding strongly to benzaldehyde (pb2A). Clusters 5 and 4 contain cells that respond to 4-methylphenol but that either are or are not inhibited by 3-octanol (pb2B and pb1B, respectively). Cluster 3 includes cells that are excited by both isoamyl acetate and 3-octanol but that show no strong response to the other odors; this cluster includes both pb3A and pb3B cells. This analysis confirms our initial observation that ORNs on the palp can reliably be assigned to cell classes that have clearly distinct response characteristics.
This cluster analysis, however, did not resolve pb3A and pb3B. Therefore we sought further evidence that pb3A and pb3B are distinguishable by testing their responses to an additional set of odors. Significant responses from pb3 cells were only observed to 3 of the initial 16 odorants (data not shown). We chose a homologous series of esters because our previous analysis had revealed a difference in the responses of pb3A and pb3B to isoamyl acetate (Fig. 6). We tested aliphatic esters (Fig.8A) with varying chain lengths of both the alcohol and acid moieties. Figure 8, Band C, shows that the responses of pb3A and pb3B are clearly distinguishable, in two respects. First, for most of the esters there is a significant difference in the absolute responses of the two neurons; for example, for pentyl acetate (5:2), the response of pb3B is 99 ± 12 spikes/sec, whereas the response of pb3A is only 9 ± 2 spikes/sec (note the different scales of they-axes in Fig. 8B,C). Second, the relative responses of the two neurons to different odors vary; for example, the pb3A neuron responds dramatically better to ethyl butyrate (2:4) than to hexyl acetate (6:2), whereas for pb3B the converse is true. This analysis confirms that the response spectra of pb3A and pb3B are clearly different and that the pb3 sensillum, like pb1 and pb2, contains two distinguishable neurons. We note with interest that for pb3A, the length of the odorant molecule correlates with potency; odors that elicit the strongest mean responses are those with a total of 6 carbons, with the next most potent being those with 4, 5, or 7 carbons, followed by those with 8 or 10 carbons. However, none of the tested odors elicits more than ∼30 spikes/sec from this neuron, leading us to suspect strongly that there are other molecules in odor space that are more effective stimuli for this neuron.
Fig. 8.
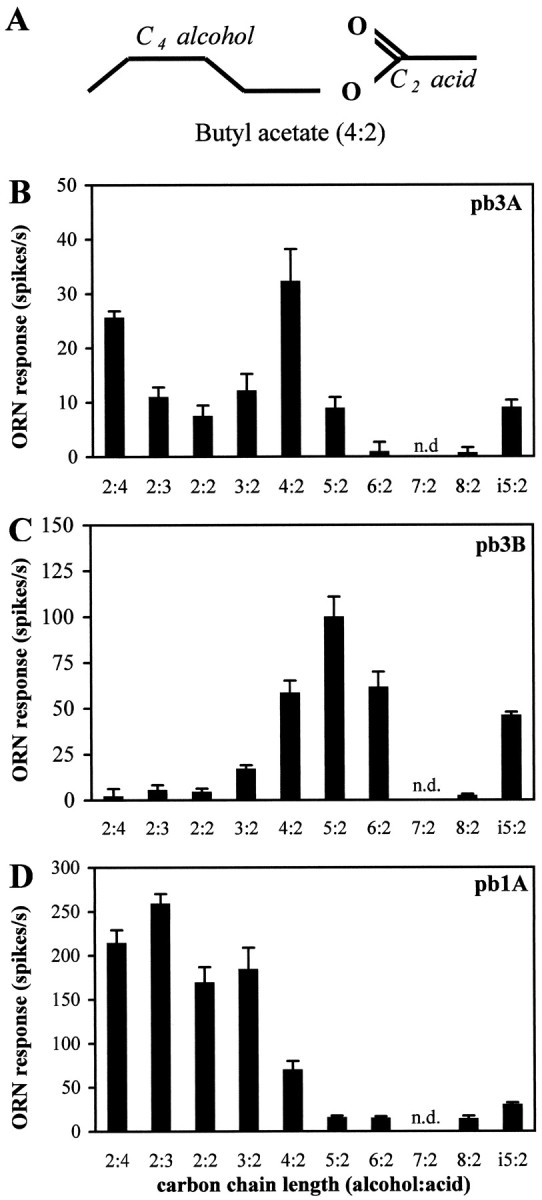
Responses of olfactory receptor neurons on theDrosophila maxillary palp to aliphatic esters confirm and characterize the distinct identities of pb3A and pb3B.A, Nomenclature illustrated with the structure of butyl acetate. i5:2 is isoamyl acetate, a branched analog of pentyl acetate (5:2). B–D, Responses of pb3A, pb3B, and pb1A, respectively, to a set of esters with varying chain lengths and structures. The indicated ORN response is measured as the increase in spikes per second over the spontaneous frequency (error bars indicate SEM; n = 8). n.d., Not done.
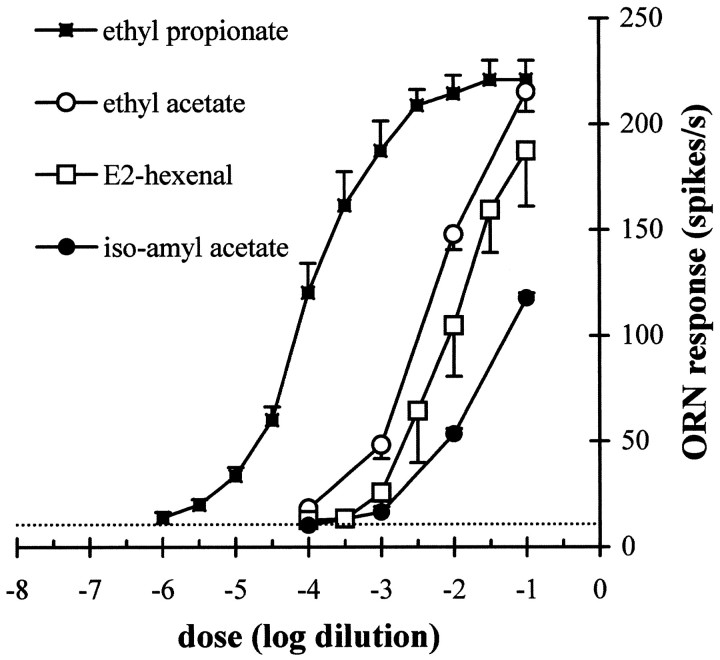
The six neuron classes differ in their response patterns; that is, odor quality may be encoded by spike-frequency differences across cell classes. However, ORNs also need to be able to encode different levels of stimuli (i.e., odor quantity). Are differences in response magnitudes constant across a range of doses of the different odors? We tested pb1A with the homologous series of esters and found that the greatest mean response was provoked by ethyl propionate (2:3) (Fig.8D). We extended this observation by generating dose–response curves (Fig. 9). The response to ethyl propionate appears as a sigmoid curve, spanning several orders of magnitude and reaching saturation at ∼225 spikes/sec. Dose–response curves for pb1A are also shown for three other odors. The differences in response magnitudes for the different esters vary widely as a function of odor concentration. When tested with 10−4 dilutions, pb1A appears narrowly tuned for ethyl propionate; at higher concentrations of odors, the differences are less striking.
Fig. 9.
Dose–response relations for pb1A to four odorants. Stimuli were presented as dilutions in 20 μl of paraffin oil on filter paper as described in Figure 4. The indicated ORN response is measured as the increase in spikes per second over the spontaneous frequency (error bars indicate SEM; n = 13). The dotted horizontal line indicates the mean response of pb1A to the paraffin oil diluent alone.
The three sensillum types have a mixed, but not random, distribution across the surface of the palp
Is there a form of topographical encoding of odor quality on the maxillary palp? The distinct response characteristics allow rapid identification of the three sensillum types. We recorded from 131 sensilla at various positions on the dorsal and lateral surfaces of one of the two palps of 43 flies, sampling almost the entire population of olfactory sensilla. There are no basiconic sensilla on the ventral and extreme proximal surfaces (Singh and Nayak, 1985). The sensillum type was easily determined using a few odors; 4-methylphenol distinguished pb1 and pb2 from pb3, and then pb1 sensilla were identified by their response to ethyl acetate, whereas pb2 sensilla were inhibited by 3-octanol. Their distribution over the surface is shown in Figure10. Their topography reveals partially overlapping distributions. Over most of the surface the three types are intermingled; thus they are not in mutually exclusive zones. However, pb2 sensilla are virtually excluded from a proximolateral region (Fig.10, arrow), indicating that the distribution of sensillum types is not random.
Fig. 10.
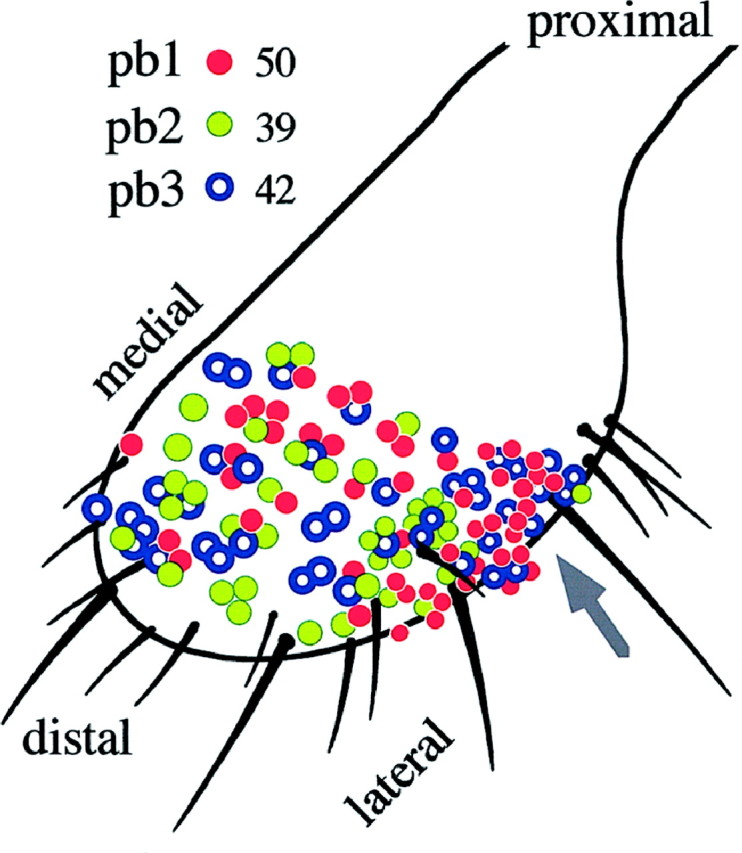
The distribution of the three sensillum types, all of which reside on the dorsal (top) and lateral surfaces of the maxillary palp. Each colored circle represents one recording from a basiconic sensillum. Clusters ofcircles indicate recordings made from sensilla in corresponding positions on different flies. The total number of recordings from each sensillum type are indicated. The arrow points to a zone where pb2 sensilla are virtually absent.
In this analysis the numbers of sensilla of each type were approximately equal: 38, 30, and 32% for pb1, pb2, and pb3, respectively (n = 131 sensilla). However, to confirm these results, we performed a second, independent experiment in which we conceptually divided the olfactory surface of the palp into five arbitrary regions of comparable area and recorded from three sensilla in each region, for each of five flies (75 sensilla total). The proportions of each sensillum type were 36 ± 2, 31 ± 3, and 33 ± 4% for pb1, pb2, and pb3, respectively.
Can the functional type of a sensillum be predicted from its coordinates on the surface of the maxillary palp? We addressed this question by analyzing a group of three sensilla that can be readily identified by virtue of their proximity to a cluster of large mechanosensory bristles on the proximolateral edge of the sensory field. Two organizational principles emerged from this analysis. First, the precise positions of individual sensilla were not fixed; the relative positions of the three sensilla to each other and their positions relative to the mechanosensory bristles varied among the four animals examined. Second, the identities of individual sensilla of this group were not the same among the four flies examined; although all three sensilla were either pb1 or pb3 in every case (the region lies within the pb2 exclusion zone), the fraction of sensilla that were of the pb1 type ranged from zero of three to three of three. These results indicate that the identity of a sensillum is not strictly determined by its position.
All three sensillum types are found on both male and female palps, and we found no evidence of sexual dimorphism of any kind. A quantitative analysis of spike frequency showed no differences between the sexes in the response of pb1 sensilla (n = 9 sensilla of each sex) to any of seven odors tested (ethyl acetate, isoamyl acetate, 4-methylphenol, benzaldehyde, 3-octanol, E2-hexenal, and cyclohexanone); limited data revealed no differences between the sexes for pb2 or pb3 sensilla. These results are consistent with the lack of sexual dimorphism in the glomeruli of the antennal lobes (Stocker, 1994) and form a striking contrast with the dimorphism observed in the olfactory sensilla and glomeruli of moths (Schneiderman and Hildebrand, 1985).
Adaptation and the independence of neurons within a sensillum
The response of an olfactory neuron may depend not only on the chemical structure (quality) and dose (quantity) of an odor stimulus but also on the previous experience of the neuron, via the process of adaptation. The effect of experience on the pb1A neuron is shown in Figure 11. First, during the course of a sustained odor stimulus, the frequency of action potentials changes (Fig. 11A). The spike frequency quickly rises to a peak of 200 spikes/sec and then rapidly declines to 25% of its peak value, over a period of 1 sec; it then slowly declines over the ensuing 24 sec to 15% of its peak value. Second, this 25 sec stimulus affects the response to a subsequent stimulus. A second stimulation with the same odor elicited a peak response only 60% of the initial peak response (Fig. 11A). The magnitude of the second response depends critically on the recovery time, that is, the time between the end of the first 25 sec stimulus and the onset of the second stimulus. The cell recovers from adaptation relatively fast (Fig. 11B), and sensitivity is approximately a linear function of the logarithm of the recovery time. After 1 sec, the responsiveness is ∼50% of that before adaptation. Full recovery is achieved after 100 sec. We note that full recovery from 500 msec stimuli is much faster than that from 25 sec stimuli (<5 sec; data not shown)
Olfactory neurons of the maxillary palp also exhibit cross-adaptation. The peak response of an unadapted pb1A to an ethyl acetate stimulus is 250 spikes/sec (Fig. 11C), but when the ethyl acetate stimulus is administered 1 sec after a prolonged stimulus of ethyl propionate, the response to ethyl acetate is reduced to 125 spikes/sec, 50% of the value for the unadapted neuron.
Having documented adaptation and cross-adaptation in these neurons, we were then in a position to investigate whether the experience of one neuron in a sensillum affects the neighboring neuron in the same sensillum. The anatomical relationship of these two neurons is intimate. Their outer dendrites are intertwined in a sensillum whose diameter is only 10 μm, and their inner dendrites and cell bodies are closely apposed, wrapped within a single sheath cell (Singh and Nayak, 1985). It has been suggested that there may be communication between adjacent neurons, perhaps mediated by the intercellular second messenger nitric oxide (Breer and Shepherd, 1993). To test the possibility of one form of intercellular communication, we stimulated pb1 sensilla with ethyl propionate, an odor that stimulates pb1A but not pb1B, and then tested whether this stimulation of pb1A affected the subsequent response of pb1B to 4-methylphenol, an odor that stimulates pb1B but not pb1A. Figure 11D shows that the pb1B neuron was unaffected by previous stimulation of pb1A; its response to 4-methylphenol was 101 ± 7% of the pb1B value before the prolonged exposure of the sensillum to ethyl propionate. By contrast, adaptation of pb1A to ethyl propionate affected the subsequent response of pb1A to ethyl propionate, ethyl acetate, E2-hexenal, and isoamyl acetate (all of which excite pb1A but not pb1B); the subsequent responses were all reduced to ±40% of the level before adaptation. We conclude that there is both adaptation and cross-adaptation within the pb1A neuron. However, this form of adaptation does not extend from the pb1A neuron to the pb1B neuron; the two neurons in a sensillum function as independent units in this respect.
DISCUSSION
We have performed a high-resolution functional analysis of an entire olfactory organ. Extensive recordings from the 60 sensilla of the Drosophila maxillary palp have revealed that its 120 neurons fall into six functional classes, each with a different response spectrum. It seems likely that all the neuronal types of this model olfactory organ have now been defined. Analysis of these neurons has revealed some basic principles of their function and organization, suggesting some of the fundamental elements with which olfactory coding can be achieved.
Odor coding in a model olfactory organ
We have defined the six neuronal classes with a panel of odors that, although chemically diverse, represents only a very small sampling of odor space. Our purpose was to distinguish among neuronal types, not to identify those ligands that elicit the strongest responses from individual neurons. ORNs in other systems have been characterized as having either broadly or narrowly tuned responses (Derby and Ache, 1984; Sicard and Holley, 1984; Boeckh et al., 1987). We have shown that in the case of the pb1A neuron the apparent breadth of tuning depends on the concentrations of odorants. When tested with odorants at higher doses (10−2 dilutions), pb1A appears to be broadly tuned (Fig. 5), but when tested with odorants at lower doses (e.g., 10−4), it appears narrowly tuned and responds only to ethyl propionate among the tested odors (Fig. 9). It is likely that there exist other odorants to which some of the cells described here, particularly pb3A, are more sensitive. We note that we do not know how the stimulus concentrations used in this study compare with those experienced in the wild. At the doses we used, some odors, such as 4-methylphenol, strongly stimulate two neuronal types. The stimulation of multiple neurons by a single odor is consistent with a model in which odor quality is assessed by integrating information from multiple neuronal types.
We have also shown that odor coding at the periphery inDrosophila involves both excitatory and inhibitory responses. One of the neuronal types, p2B, exhibits two modes of response: it is excited by some stimuli and inhibited by others. Moreover, the extent of inhibition varies with different odor stimuli. It seems likely that the excitation and inhibition that we have observed reflect depolarizing and hyperpolarizing ion currents in the dendrites of the ORNs. The occurrence of such responses in an individual ORN has been demonstrated in insects (Boeckh, 1967; Dubin and Harris, 1997) and crustaceans (Michel and Ache, 1994). In the latter, depolarizing and hyperpolarizing currents are mediated via the IP3 and cAMP transduction pathways, respectively (Ache and Zhainazarov, 1995). The existence of two modes of response suggests the possibility that multiple messages can be sent from the same neuron; it provides an additional degree of freedom and expands the possible means by which odor coding can be achieved with a limited number of sensory neurons.
Not only can an individual neuron be excited by one odor and inhibited by another, but an individual odor can excite one cell and inhibit another. For example, benzaldehyde stimulates pb2A but inhibits pb2B. The simultaneous production of opposite effects on two neurons may provide a means of generating contrast, which may enhance the recognition of specific odors.
We have also found that different odors can have different effects on the kinetics of the response. For example, firing of the pb1b and pb2b neurons stimulated with 4-methylphenol continues long past the end of the stimulus (Fig. 4B,C), whereas firing of other neurons with other odors terminates at the end of the stimulus period (Fig. 4A,E). Phenols also elicit prolonged firing of receptor cells in tsetse fly antennae (Den Otter and Van der Goes van Naters, 1993), but such extended tonic responses are not limited to phenols; they have also been observed in moth neurons after stimulation with pheromones (Almaas et al., 1991). Thus the temporal pattern of action potentials provides another degree of freedom: the time course of spikes may contain information aiding in odor recognition.
Neural organization of the maxillary palp and its development
The number of functional types of neurons on the maxillary palp, six, is of the same order as the estimated number of glomeruli that receive afferent fibers from it: five, as reported by Singh and Nayak (1985), or three, as estimated by Stocker (1994). This approximate numerical equivalence, in which the number of neuronal classes is determined by direct physiological analysis, is consistent with the approximate equivalence of the number of glomeruli with the number of neuronal types in the mammalian main olfactory epithelium, in which the number of neuronal types is estimated on the basis of an analysis of receptor gene expression (Mombaerts et al., 1996). We note that in moths, there is also a well documented pattern of projection from particular functional classes of neurons, pheromone-sensitive neurons, to a cluster of specialized glomeruli, the macroglomerular complex (Hansson et al., 1992; Hildebrand and Shepherd, 1997). However, it remains to be seen whether the two neurons in each sensillum type project to one glomerulus, giving three palpal glomeruli, or to two glomeruli, which would give six palpal glomeruli. The total number of olfactory glomeruli reported for the antennal lobe ofDrosophila is ∼40 (Laissue et al., 1999). Hence the total peripheral input to the olfactory-processing centers in the CNS may consist of ≤ 40 basic types of input elements.
A major way in which the insect olfactory system differs from that of vertebrates is that the neurons of the sensory field are compartmentalized in sensilla. The insect olfactory system can in this respect be considered as a “compound nose,” by analogy to the compound eye (Hekmat-Scafe et al., 1998). We have found that neurons of the palp are ordered with respect to this level of organization; the six types of neurons are distributed within sensilla in stereotyped pairs, with a neuron of one particular response spectrum cohabiting in a sensillum with a neuron of another particular response spectrum. Despite the intimate cohabitation of neurons within a sensillum, our cross-adaptation experiments show that they are able to function primarily as independent units of perception. This independence and their distinct response spectra are observed despite the fact that both neurons in a sensillum share a common pool of binding proteins (Hekmat-Scafe et al., 1997) and a common electrical circuit (Kaissling, 1987). In this sense, olfactory tissues in insects may be similar to those in mammals.
The largely overlapping distribution of the three sensillum types across the sensory field shows that in the maxillary palp there is not an obvious odotopic layout at the primary neuron level. However, the presence of a pb2 “exclusion” zone provides some heterogeneity and likely points to zones that are developmentally distinct. Our analysis of sensillum function and organization raises interesting questions about how such a sensory field develops. Olfactory sensilla develop from founder cells (Ray and Rodrigues, 1995) that build different sensillum categories. The mixed distribution of functional types on the palp and the variability in the positions of individual sensilla suggest that sensilla are not committed to one of the three alternative fates strictly according to their position in the field. We do not know how the expression of genes encoding the choice between pb1, pb2, and pb3 is regulated. Moreover, further studies will be necessary to reveal the developmental logic by which the stereotyped pairing of neurons is produced. What is the mechanism that regulates expression of class-specific elements such as receptors and coordinates it between the two neurons of one sensillum?
The palp as an accessory olfactory organ
The olfactory system of adult Drosophila contains two organs, the antenna and the maxillary palp. Each antenna is covered with ∼500 sensilla of three morphological types, whereas the maxillary palp is covered with 60 sensilla of a single morphological type. It is tempting to compare this arrangement with the presence of two olfactory organs in mammals, the main olfactory epithelium (MOE) and the vomeronasal organ (VNO). Like the VNO, the maxillary palp has been associated with pheromone response and the modulation of sexual behavior (Stocker and Gendre, 1989). The VNO neurons project to an accessory olfactory bulb that does not overlap with the main olfactory bulb (Farbman, 1992; Dulac and Axel, 1995); likewise, projections from the palp have been reported to map to a subset of glomeruli distinct from those that receive input from the antenna (Stocker, 1994; cf.Singh and Nayak, 1985). However, VNO neurons differ from MOE neurons in that they are microvillous rather than ciliate (Farbman, 1992), whereas both palpal and antennal ORNs are ciliate (Singh and Nayak, 1985). In addition, the VNO neurons probably perceive a different type of odorants (less volatile and larger molecules) from those perceived by the MOE, whereas the present study shows that the maxillary palp is sensitive to many small, volatile molecules that also stimulate the antenna (this study) (Siddiqi, 1991; Ayer and Carlson, 1992). We also have found no evidence of a special role of the palp in pheromone perception. First, cis-vaccenyl acetate, a compound reported previously as an inhibitory sex pheromone (Jallon, 1984), induces a response from pb1A. However, this neuron responds more strongly to many other odors, and cis-vaccenyl acetate has also been found to stimulate an antennal neuron (Clyne et al., 1997). Second, we did not find differences between male and female ORN populations, making it unlikely that sex-specific pheromonal receptor cells occur on the palps.
In summary, we have provided an extensive description of an olfactory organ, compiling what is likely a complete catalog of its neural elements. We have shown that olfactory information in this organ is encoded by a limited number of ORN classes. Furthermore, this study, taken together with previous studies (Siddiqi, 1991; Clyne et al., 1997), suggests that the total number of ORN response classes in the entire olfactory system is limited and identifiable. Although a detailed roster of olfactory neurons of the worm Caenorhabditis elegans has been made available via behavioral analysis (Bargmann et al., 1993), it has not been possible to identify exhaustively all the neuronal types of olfactory systems of higher organisms on account of their complexity. Physiological analysis of the receptor neurons of the Drosophila palp has also revealed a rich diversity of response dynamics, which together with the existence of neuron classes establishes a basis for olfactory coding. In addition to principles of functional organization, the present study provides a foundation for analysis of the molecular and genetic mechanisms that underlie the function and development of this model olfactory organ.
Footnotes
This work was supported by the Human Frontier Science Program Fellowship LT 277/97 to M.d.B., by a National Science Foundation predoctoral fellowship to P.J.C., and by National Institutes of Health Grant DC-02174 and a grant from the Human Frontier Science Program to J.R.C.
Correspondence should be addressed to Dr. John R. Carlson, Department of Molecular, Cellular, and Developmental Biology, Yale University, Kline Biology Tower, P.O. Box 208103, New Haven, CT 06520-8103.
REFERENCES
- 1.Ache BW, Zhainazarov A. Dual second-messenger pathways in olfactory transduction. Curr Opin Neurobiol. 1995;5:461–466. doi: 10.1016/0959-4388(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 2.Almaas TJ, Christensen TA, Mustaparta H. Chemical communication in heliothine moths. I. Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J Comp Physiol [A] 1991;169:249–258. [Google Scholar]
- 3.Altner H, Prillinger L. Ultrastructure of invertebrate chemo-, thermo- and hygroreceptors and its functional significance. Int Rev Cytol. 1980;67:69–139. [Google Scholar]
- 4.Anderberg MR. Cluster analysis for applications. Academic; New York: 1973. [Google Scholar]
- 5.Anderson P, Hansson BS, Löfqvist J. Plant-odour-specific receptor neurones on the antennae of female and male Spodoptera littoralis. Physiol Entomol. 1995;20:189–198. [Google Scholar]
- 6.Ayer R, Carlson JR. Olfactory physiology in the Drosophila antenna and maxillary palp: acj6 distinguishes two classes of odorant pathways. J Neurobiol. 1992;23:965–982. doi: 10.1002/neu.480230804. [DOI] [PubMed] [Google Scholar]
- 7.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 8.Bieber SL, Smith DV. Multivariate analysis of sensory data: a comparison of methods. Chem Senses. 1986;11:19–47. [Google Scholar]
- 9.Boeckh J. Inhibition and excitation of single insect olfactory receptors, and their role as a primary sensory code. In: Hayashi T, editor. Olfaction and taste. Pergamon; Oxford: 1967. pp. 721–735. [Google Scholar]
- 10.Boeckh J. Chemoreceptors: their structure and function. In: Laverack MS, Cosens PJ, editors. Sense organs. Blackie; London: 1981. pp. 86–99. [Google Scholar]
- 11.Boeckh J, Ernst K-D, Selsam P. Neurophysiology and neuroanatomy of the olfactory pathway in the cockroach. In: Roper SD, Atema J, editors. Olfaction and taste, Vol IX. New York Academy of Sciences; New York: 1987. pp. 39–43. [DOI] [PubMed] [Google Scholar]
- 12.Breer H, Shepherd GM. Implications of the NO-cGMP system for olfaction. Trends Neurosci. 1993;16:5–9. doi: 10.1016/0166-2236(93)90040-s. [DOI] [PubMed] [Google Scholar]
- 13.Buck LB. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- 14.Buck LB, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 15.Bursell E, Gough AJE, Beevor PS, Cork A, Hall DR, Vale GA. Identification of components of cattle urine attractive to tsetse flies, Glossina spp. (Diptera: Glossinidae). Bull Entomol Res. 1988;78:281–291. [Google Scholar]
- 16.Carlson JR. Olfaction in Drosophila: from odor to behavior. Trends Genet. 1996;12:175–180. doi: 10.1016/0168-9525(96)10015-9. [DOI] [PubMed] [Google Scholar]
- 17.Clyne P, Grant AJ, O’Connell RJ, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invertebrate Neurosci. 1997;3:127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- 18.Den Otter CJ, Van der Goes van Naters WM. Responses of individual olfactory cells of tsetse flies (Glossina m. morsitans) to phenols from cattle urine. Physiol Entomol. 1993;18:43–49. [Google Scholar]
- 19.Derby CD, Ache BW. Quality coding of a complex odorant in an invertebrate. J Neurophysiol. 1984;51:906–924. doi: 10.1152/jn.1984.51.5.906. [DOI] [PubMed] [Google Scholar]
- 20.Dubin AE, Harris GL. Voltage-activated and odor-modulated conductances in olfactory neurons of Drosophila melanogaster. J Neurobiol. 1997;32:123–137. doi: 10.1002/(sici)1097-4695(199701)32:1<123::aid-neu11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 22.Farbman AI. Cell biology of olfaction. Cambridge UP; Cambridge, UK: 1992. [Google Scholar]
- 23.Fujishiro N, Kijima H, Morita H. Impulse frequency and action potential amplitude in labellar chemosensory neurons of Drosophila melanogaster. J Insect Physiol. 1984;30:317–325. [Google Scholar]
- 24.Getz WM, Akers RP. Response of American cockroach (Periplaneta americana) olfactory receptors to selected alcohol odorants and their binary combinations. J Comp Physiol [A] 1997;180:701–709. [Google Scholar]
- 25.Guillet JC, Bernard J. Shape and amplitude of the spikes induced by natural or electrical stimulation in insect receptors. J Insect Physiol. 1972;18:2155–2171. [Google Scholar]
- 26.Hansson BS. Olfaction in Lepidoptera. Experientia. 1995;51:1003–1027. [Google Scholar]
- 27.Hansson BS, Ljungberg H, Hallberg E, Löfstedt C. Functional specialization of olfactory glomeruli in a moth. Science. 1992;256:1313–1315. doi: 10.1126/science.1598574. [DOI] [PubMed] [Google Scholar]
- 28.Hansson BS, Hallberg E, Löfstedt C, Steinbrecht RA. Correlation between dendrite diameter and action potential amplitude in sex pheromone specific receptor neurons in male Ostrinia nubilalis (Lepidoptera: pyralidae). Tissue Cell. 1994;26:503–512. doi: 10.1016/0040-8166(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 29.Hekmat-Scafe DS, Steinbrecht RA, Carlson JR. Coexpression of two odorant-binding protein homologs in Drosophila: implications for olfactory coding. J Neurosci. 1997;17:1616–1624. doi: 10.1523/JNEUROSCI.17-05-01616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hekmat-Scafe DS, Steinbrecht RA, Carlson JR. Olfactory coding in a compound nose, coexpression of odorant-binding proteins in Drosophila. In: Murphy C, editor. Olfaction and taste, Vol XII, An international symposium. New York Academy of Sciences; New York: 1998. pp. 311–315. [DOI] [PubMed] [Google Scholar]
- 31.Helfand SL, Carlson JR. Isolation and characterization of an olfactory mutant in Drosophila with a chemical specific defect. Proc Natl Acad Sci USA. 1989;86:2908–2912. doi: 10.1073/pnas.86.8.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- 33.Jallon J-M. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet. 1984;14:441–478. doi: 10.1007/BF01065444. [DOI] [PubMed] [Google Scholar]
- 34.Kaissling K-E. R. H. Wright lectures on insect olfaction. Simon Fraser University; Burnaby, British Columbia, Canada: 1987. [Google Scholar]
- 35.Kaissling K-E. Single unit and electroantennogram recordings in insect olfactory organs. In: Spielman AI, Brand JG, editors. Experimental cell biology of taste and olfaction, current techniques and protocols. CRC; Boca Raton, FL: 1995. pp. 361–377. [Google Scholar]
- 36.Laissue PP, Reiter C, Hiesinger PR, Halter S, Fishbach K-F, Stocker RF. 3D reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- 37.Loftus R, Corbière-Tichané G. Antennal warm and cold receptors of the cave beetle, Spheophyes lucidulus Delar., in sensilla with a lamellated dendrite. I. Response to sudden temperature change. J Comp Physiol [A] 1981;143:443–452. [Google Scholar]
- 38.Michel WC, Ache BW. Odor-evoked inhibition in primary olfactory neurons. Chem Senses. 1994;19:11–24. doi: 10.1093/chemse/19.1.11. [DOI] [PubMed] [Google Scholar]
- 39.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 40.Ray K, Rodrigues V. Cellular events during development of olfactory sense organs in Drosophila melanogaster. Dev Biol. 1995;167:426–438. doi: 10.1006/dbio.1995.1039. [DOI] [PubMed] [Google Scholar]
- 41.Riesgo-Escovar JR, Piekos WB, Carlson JR. The maxillary palp of Drosophila: ultrastructural and physiology depends on the lozenge gene. J Comp Physiol [A] 1997;180:143–150. doi: 10.1007/s003590050035. [DOI] [PubMed] [Google Scholar]
- 42.Rumbo ER. The shape of extracellularly recorded nerve impulses from pheromone receptors. Chem Senses. 1989;14:361–369. [Google Scholar]
- 43.Schneiderman AM, Hildebrand JG. Sexually dimorphic development of the insect olfactory pathway. Trends Neurosci. 1985;8:494–499. [Google Scholar]
- 44.Sicard G, Holley A. Receptor cell responses to odorants: similarities and differences among odorants. Brain Res. 1984;292:283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqi O. Olfaction in Drosophila. In: Wysocki CJ, Kare MR, editors. Chemical senses, Vol 3, Genetics of perception and communications. Dekker; New York: 1991. pp. 79–96. [Google Scholar]
- 46.Singh RN, Nayak SV. Fine structure and primary sensory projections of sensilla on the maxillary palp of Drosophila melanogaster Meigen (Diptera: Drosphilidae). Int J Insect Morphol Embryol. 1985;14:291–306. [Google Scholar]
- 47.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 48.Stocker RF, Gendre N. Courtship behavior of Drosophila genetically or surgically deprived of basiconic sensilla. Behav Genet. 1989;19:371–385. doi: 10.1007/BF01066165. [DOI] [PubMed] [Google Scholar]
- 49.Taneja J, Guerin PM. Ammonia attracts the haematophagous bug Triatoma infestans: behavioural and neurophysiological data on nymphs. J Comp Physiol [A] 1997;181:21–34. [Google Scholar]
- 50.Visser JH. Host odour perception in phytophagous insects. Annu Rev Entomol. 1986;31:121–144. [Google Scholar]
- 51.Ward JH., Jr Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. [Google Scholar]