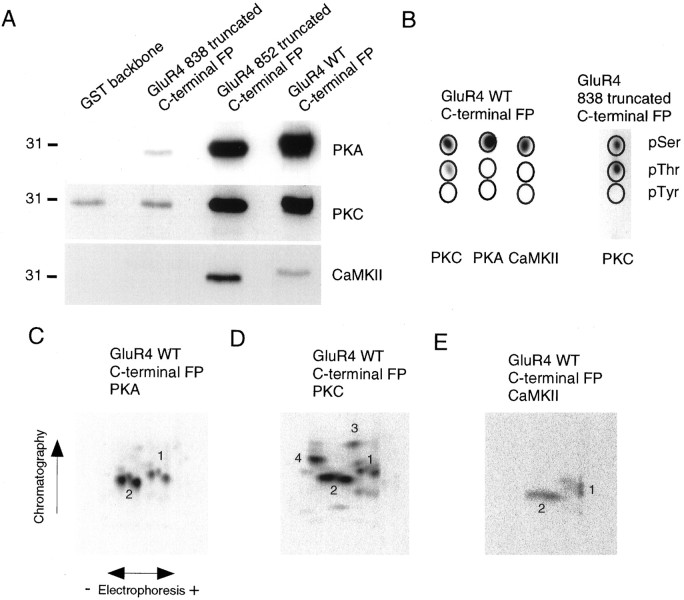
Fig. 3.
Phosphorylation of GluR4 fusion proteins.A, Bacterial fusion proteins containing GluR4 C terminal or partial segments of GluR4 C terminal (amino acids 815–838 or 815–852) were incubated with purified kinases, as indicated, in the presence of [γ-32P] ATP, resolved by SDS-PAGE, and analyzed by autoradiography. B, Phosphoamino acid analysis of phosphorylated GluR4 fusion proteins. Phosphoamino acid analysis was performed as described in Figure 1B.C–E, The phosphorylated GluR4 C-terminal fusion proteins were excised from the gel and digested with trypsin; the phosphopeptides were resolved in two dimensions in TLC plates and visualized by autoradiography. In every case the phosphopeptide map for the fusion protein containing amino acids 815–852 of GluR4 protein was identical to that for the full-length C-terminal GluR4 fusion protein.